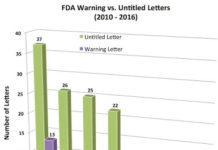
2016 Sees a Slight Uptick in FDA’s Enforcement Actions
UPDATED 28 December 2016: On December 21, 2016, FDA issued two new Untitled letters for a total of 11 letters in 2016. Is FDA getting it's last licks in? More about...
Can This Work for Pharma? The Continual Quest of Creative Advertisers
PM360 magazine is asking its readers find marketing campaigns and tactics used by consumer packaged goods, retail, and other industries to give pharma marketers some fresh ideas. Here's what one ad...
FDA Compliant Diclegis Instagram Promo by (Good) Emily Maynard Johnson Can’t Compete with (Bad)...
About 22 weeks ago, Emily Maynard Johnson, who appeared on ABC's The Bachelor and The Bachelorette, posted the following Diclegis promotion to her Instagram account:
I assume you've noticed that all the...
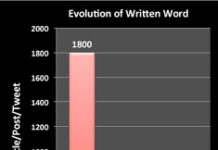
The Evolution of the Written Word in the Social Media Information Age
I started writing about the drug industry – specifically the commercial side of the industry – in 2002 with the publication of the first issue of Pharma Marketing News. At the...
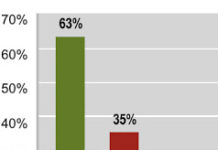
FDA Hears Results of Two Different Off-Label Surveys of Consumers at Recent Public Hearing
At the recent Part 15 FDA hearing on off-label promotion (see here) results from two different consumer/patient surveys were presented. The first was presented by John Mack (i.e., me), Editor of...
What is “Truthful and Non-misleading?” That is the Question FDA Must Answer to Allow...
Yesterday, I attended and presented at FDA's Public Hearing on drug company communications regarding unapproved uses of drugs. My presentation had to do with off-label promotion directed at consumers and patients...
This Little Piggy… A New Pharma Nursery Rhyme
I attended the 10th Annual Digital Pharma East conference in Philadelphia last week. One of the highlights for me was the "after party" hosted by Intouch Solutions at the Reading Terminal...
The Pharma Digital Health Accelerator Club
My friend Fard Johnmar, founder and president of Enspektos, recently identified a few issues facing the drug industry as it tries keep up with digital innovation in the health field:
Pharmaceutical executives...
FDA Will Apply the “Uncanny Valley” Hypothesis to Test the “Eeriness” of Animated Characters...
AbbVie, Astrazeneca, Eli Lilly, GSK, Merck, Regeneron Pharmaceuticals, plus others have submitted comments to the FDA regarding its plans to research animated spokes-characters in DTC Drug Ads (see Federal Register Docket...
Meet Me at FDA Public Hearing on Off-Label Promotion on November 9, 2016
I will be Speaking at FDA's Part 15 hearing on "Manufacturer Communications Regarding Unapproved Uses of Approved or Cleared Medical Products" on 9 November, 2016 (read "FDA May Have No Choice...







![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)



