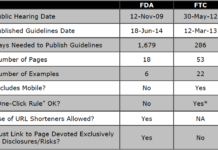
The “Risk-Only” Landing Page Requirement: Can We Get FDA to Adopt FTC’s Thinking and...
One of FDA's Ten Commandments -- the FIRST one, in fact -- states:
You shall have no other regulatory agencies before Me.
Several people have asked me, "John, what other agency could pharma...
Even Though There Are Fewer Sales Reps, More Physicians Deny Rep Access
According to the spring 2014 AccessMonitor™ report from global sales and marketing consulting firm ZS Associates, pharmaceutical access to physicians continues to decline. Only 51% of physicians/prescribers now allow access to...
Why is PhRMA Defending Gilead’s Sovaldi Pricing?
In an article published in The Hill, Lori Reilly, vice president for policy and research at the Pharmaceutical Research and Manufacturers of America (PhRMA), whose job is to develop federal legislative,...
FDA’s Ten Commandments
Remember FDA's Social Media Webinar?
It was supposed to answer all our questions about the two recent social media guidances issued by the FDA.
There were technical difficulties and when participants could actually...
Do Marketing Buzzwords Affect Pharma’s Reputation Among Patients & Physicians?
Consultant and strategist Andrew Spong, PhD, former scholar and currently STweM‘s managing director, recently tweeted:
"Building trust is far from impossible for #pharma. Abstaining from digital conferences is a great start."
The tweet...
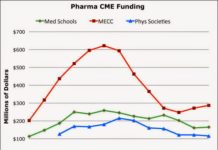
Total CME Revenue is Up, But Pharma Support is Down (Again) in 2013
The Accreditation Council for Continuing Medical Education (ACCME) 2013 Annual Report is out. Here's the data regarding total CME income and breakdown according to source of income. Note: the data analyzed...
FDA’s Social Media Guidance Webinar: A Third Guidance Needed for Mobile Devices?
Yesterday, the FDA hosted a public webinar to "further" the Agency's "communications with stakeholders, media and the public" about recently-published social media guidelines.
Unfortunately, I did not learn of this until it...
Has Gilead’s Director of Regulatory Affairs Been Hiding Under a Rock Since 2009? Will...
At the end of June, the FDA sent a Notice of Violation (NOV) letter to Dr. C (you can find his name in the letter), Director, Regulatory Affairs Advertising and Promotion...
Another Idea for Creating FDA-Compliant Promotional Tweets: Put the Carriage Before the Horse
In previous posts, I discussed a few different techniques that pharma marketers could use to create Rx branded promotional tweets that satisfy recently-published FDA guidelines.
One such idea was to attach an...
Will Twitter’s Card Technology Allow Pharma Brands to Post FDA-Compliant Promotional Tweets?
In today's Pharma Marketing News, I reviewed three "workarounds" that might allow pharma marketers to post promotional tweets that satisfy FDA's recently published (18 June 2014) guidelines:
Chain of Tweets
Tweets with Images...








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)



