“Innovative” Pharma Marketing Is Not Viral
Several recent articles and blog posts have claimed there is a lack of "Innovation" among pharma marketers who are afraid to "rock the boat."
World of DTC Marketing blogger Rich Meyer, for...
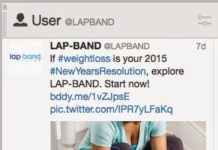
LAP-BAND’s Social Media Campaign Definitely Violates FDA Guidelines
I heard that a number of participants at the 33rd Annual J.P. Morgan Healthcare Conference in San Francisco were "baffled" by LAP-BAND's social media campaign. LAP-BAND, marketed by Apollo Endosurgery, is...
Are Online Peer-to-Peer Physician Discussions Overrated?
Congrats to Thibaud Guymard (@thibaudguymard), Digital Marketing Manager at MSD France, and his Comuniti team for winning the Prix Or de la Communication Médicale & Hospitalière in Paris at the...
FDA’s 2015 Guidance Agenda – Just Like the 2014 Agenda – Promises Publication of...
FDA/CDER has published its 2015 Guidance Agenda (here). Here's what's under the Advertising Category:
Brief Summary and Adequate Directions for Use: Disclosing Risk Information in Consumer-Directed Print Advertisements and Promotional Labeling for...
2014 Was Another Record Year for FDA: Fewest Number of Untitled/Warning Letters Ever!
In 2014, the FDA approved 41 new drugs -- the most since 1996 (see here). That's quite a "record" even if 37% of the approvals were made in December alone.
But FDA...
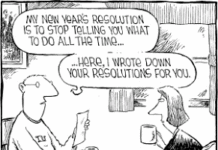
2015 New Year’s Resolutions for Pharma Marketers
My #1 New Year's Resolution is the same every year:
Stop telling pharmaceutical marketers what to do all the time.
Unfortunately, I have never kept that resolution. So, here's my list of 2015...
Critique of an Ad Seen in “Modern Pharma Guidance” Magazine
The ad shown on the left is the subject of an FDA/OPDP "untitled letter" (definition), which took aim at a Sunovion professional print ad for seizure med Aptiom.
According to the FDA...
Pharma: The Year 2014 in Images
These are my favorite images and posts that appeared in Pharma Marketing Blog in 2014 (see embedded slide deck below - you can also view this directly on slideshare here).
It was...
Et Tu, Amgen? Blincyto’s Bling! Bling! Price Tag!
FDA approved Amgen's Blincyto (blinatumomab) on December 3, 2014, as a second-line treatment for Philadelphia chromosome-negative precursor B-cell acute lymphoblastic leukemia, which affects around 6,000 patients in the US.
The price tag...
Pfizer’s “Humorous” Menopause Ad Mentions the “V” Word
Pfizer marketers have done it again! First it was an all-female Viagra ad campaign for the treatment of ED (erectile dysfunction; read "Oh Yeah, Baby! Show Me More!... Viagra TV Ads...










![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)



