Dear Patient, Come to the “Dark Side” & Engage with Pharma
Engaging patients was a major theme of the eyeforpharma Barcelona 2015 conference (hashtag e4pbarca), which just closed out after 3 days of presentations with a keynote presentation by Dr. Anne Beal,...
Scary Pharma/Drug Ads in Medical Journals
Drug print ads aimed at patients are usually upbeat showing the benefits of the pill. Print ads intended for physicians on the other hand are often dark and downright scary. I...
Attending #e4pbarca Remotely: Second Day
There's been a lot of tweeting during the eyeforpharma Barcelona conference yesterday and today. I summarized a few of the issues being discussed that were of interest to me during yesterday's...
Attending #e4pbarca Remotely
I'm getting up early to attend the 13th Annual eyeforpharma Barcelona 2015 conference from the comfort of my home office.
Disclosure: eyeforpharma is a client of mine - Pharma Marketing News is...
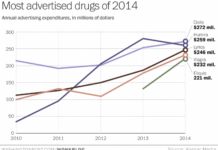
DTC Ad Spend is Back Stronger Than Ever, Baby!
OK, maybe that's an exaggeration, but according to Wonkblog, a blog published by the Washington Post, and based on data from Kantar Health, "drug companies are bombarding your TV with more...
FDA’s Reprint Distribution Guidelines are “Counterintuitive” & “Indecent”
FDA's Reprint Distribution Guidelines are "Counterintuitive" & "Indecent"
A Pig, a Cow, a Robot, an FDA Warning Letter
In a March 5, 2015, Warning Letter to Discovery Laboratories, Inc. (here), regarding its Surfaxin promotional website, FDA cited "evolution" as a false claim of superiority. Well, not exactly.
FDA noted the...
A Day in the Life of a Typical Digital Marketer vs. My Day Yesterday
I was sent an email from a reader of Pharma Marketing Blog directing me to an infographic about the day in the life of a digital marketer. You can view the...
FDA Develops a Mobile App for Reporting Drug Shortages. But Wouldn’t an App for...
FDA/CDER launched its first and only mobile app on iTunes: DrugShortages (see logo on left).
The iTunes blurb suggests the public would find this information important:
"Access to drug shortage information is now...
In a Major Turnaround, FDA Now Requires “Low-T” Drug Label Changes & Safety Studies:...
FDA characterizes today's Safety Announcement requiring label changes for drugs that treat "low testosterone" merely "an update to the FDA Drug Safety Communication: FDA Evaluating Risk of Stroke, Heart Attack, and...










![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)



