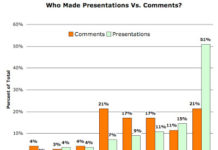
Consumer Advocates Step Up and Submit Comments to FDA Regarding Regulation of Social Media
The deadline has come and gone for submitting comments to docket FDA-2009-N-0441 regarding "Promotion of FDA-Regulated Medical Products Using the Internet and Social Media Tools." You can find ALL of the...
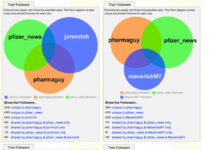
According to Follower Wonk, I Have More Followers in Common with Pfizer Than Other...
This morning I learned about "Follower Wonk," a neat Twitter application that creates Venn diagrams showing the overlap among followers of up to three different Twitter accounts. It does other stuff,...
Where’s Your Social Media Crisis Management Plan?
According to Chris Kenton of SmartMarketers.com's Inspire Blog, "Surprisingly few companies... have protocols in place to manage social media disasters as they unfold -- even those that have sophisticated crisis management...
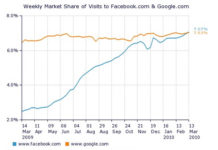
Drug Companies Are Flocking to Facebook for Eyeballs, Not Conversation
More and more pharmaceutical companies are launching Facebook pages that promote their products. The latest is Allergan, which launched a celebrity Facebook campaign for Juvederm, a dermal filler used for "long-lasting...
Johnson & Johnson Urges FDA to Be As Flexible As FTC in Its Regulation...
In comments to the FDA, Johnson & Johnson (JNJ) stated "Flexibility in regulatory approach is crucial in the evolving digital world." The comment was part of an 8-page document submitted to...
Novartis Proposes Use of Unique FDA-Approved Short URLs & Hashtags to Overcome Space Limitation...
Novartis submitted 6 pages of comments to Docket No. FDA‐2009‐N‐0441 regarding Promotion of FDA‐Regulated Medical Products Using the Internet and Social Media Tools (find it here).
Novartis made many interesting proposals on...
Should Sanofi-Aventis Submit an Adverse Event Report Based on “Disgruntled Patient’s” Comments to VOICES...
Sanofi-Aventis (S-A) submitted 14 pages of comments to Docket No. FDA‐2009‐N‐0441 regarding Promotion of FDA‐Regulated Medical Products Using the Internet and Social Media Tools (find it here).
"As part of routine monitoring...
Sanofi-Aventis Updates Facebook Site with Disclaimer, But Shirley Still Posting About Her Side Effects
Recently, Sanofi-Aventis has been having problems with its VOICES Facebook page (see "Disgruntled Patient Shuts Down sanofi-aventis Facebook Page"). I previously suggested S-A made a faux pas because it did NOT...
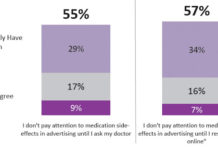
Yahoo!’s Claim That Patients Are “Ambivalent” When It Comes to Side Effect Information in...
Yahoo! submitted comments to Docket No. FDA‐2009‐N‐0441 regarding Promotion of FDA‐Regulated Medical Products Using the Internet and Social Media Tools (find it here).
In defense of the "one-click rule" for space-limited "generic"...
Use of Internet to Obtain Health Information Can Make You Depressed
FDA is considering issuing new guidelines regarding regulation of pharmaceutical use of the Internet for promoting drugs. The drug industry and online service providers like Google and Yahoo! hammered into the...






![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)



