A Pharma Social Media Conspiracy Theory: Were Guidelines Held Hostage as Part if FDA’s...
Were FDA's infamous 14 warning letters to pharma a ploy to force Google into a $500M DOJ settlement regarding illegal online pharmacy ads? In addition, could FDA have been holding back issuing pharma social media guidelines -- which would include guidelines for displayig compliant information in space-limited applications such as Twitter AND Google Adwords -- until Google settled its case with the DOJ?
FDA Drops Social Media from Its Guidance Agenda: Focuses Instead on Responsding to “Unsolicited”...
FDA's revised draft guidance calendar for year 2011 is missing 'Promotion of Prescription Drug Products Using Social Media Tools,
Pharma Marketing News Vol. 10, No. 11: 21 June 2011
Welcome to Volume 10, Issue #11 (21 JUNE 2011) of Pharma Marketing News.
Pharma Marketing News Vol. 10, No. 9: 18 May 2011
Welcome to Volume 10, Issue #9 (18 MAY 2011) of Pharma Marketing News.
Pharma Marketing News Vol. 10, No. 9: 18 May 2011
Welcome to Volume 10, Issue #9 (18 MAY 2011) of Pharma Marketing News.
Pharma SmartPhone/Tablet Apps: Is There a Regulation for That?
Pharmaceutical marketers have jumped on the mobile application bandwagon as more and more companies roll out health-related applications for smartphones and touchpads such as Apple's iPhone and iPad. But will the FDA regulate certain smartphone apps as medical devices? This article discusses the regulatory and other issues associated with pharma developed or sponsored health apps.
Pharma SmartPhone/Tablet Apps: Is There a Regulation for That?
Pharmaceutical marketers have jumped on the mobile application bandwagon as more and more companies roll out health-related applications for smartphones and touchpads such as Apple's iPhone and iPad. But will the FDA regulate certain smartphone apps as medical devices? This article discusses the regulatory and other issues associated with pharma developed or sponsored health apps.
Challenges of Regulating Mobile Medical Apps: Legal and Regulatory Issues You Should Know About
A conversation with Joseph Kim, MD, MPH, VP of Medical Affairs and Technology at Medical Communications Media, Inc., about the rapid development of mobile medical applications and the legal and regulatory issues that physicians, patients, and pharma developers/sponsors should be aware of.
Consequences of eDetailing Technology
The Death of the Traditional Pharma Salesman and the Birth of the 'Sales Cyborg.' eDetailing in one form or another seems to be making a comeback since the recession hit the drug industry in 2007 and 2008. Which is the 'chicken' and which is the 'egg'? Did the uptick in adoption of eDetailing technology lead to the recent layoff of pharma reps or were reps laid off because of the economy and subsequently replaced by machines?
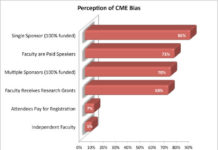
Physicians Are Concerned About Pharma Support of CME, But Are Unwilling to Pay Their...
Talk about having your cake and eating it too! Commercial funding of continuing medical education (CME) and the potential for bias appear to concern many physicians, yet only a TINY MINORITY (7%) are willing to pay registration fees to eliminate or offset commercial funding sources, according to a report in the May 9, 2011, issue of Archives of Internal Medicine, one of the JAMA/Archives journals.





![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)



