Brits Beat FDA and PhRMA: Issue Social Media Guidance for Pharma
Prescription Medicines Code of Practice Authority Issues Social Media Guidance for Pharma. The Brits have won the race to issue social media guidance for the drug industry! In April, 2011, the Prescription Medicines Code of Practice Authority (PMCPA), which oversees the self-regulatory code of the Association of the British Pharmaceutical Industry (ABPI), published 'informal guidance' providing the drug industry advice on how to use online communications.
Pharma Marketing News Vol. 10, No. 7: 15 April 2011
Welcome to Volume 10, Issue #7 (15 APRIL 2011) of Pharma Marketing News.
Pharma Marketing News Vol. 10, No. 7: 15 April 2011
Welcome to Volume 10, Issue #7 (15 APRIL 2011) of Pharma Marketing News.
The Changing Role of Pharma Sales Reps
Pharma companies need to drive maximum value out of products and they can't do that with a 'one-size-fits-all' cookie-cutter approach to deploying field forces. Brand managers will look to outsourced providers to take advantage of the expertise these providers can bring to the table. This article is a summary of an interview of Nancy Lurker, CEO of PDI, Inc., which provides promotional outsourced services to healthcare companies.
Pharma Marketing News Vol. 10, No. 6: 29 MARCH 2011
Welcome to Volume 10, Issue #6 (30 MARCH 2011) of Pharma Marketing News.
Pharma Marketing News Vol. 10, No. 6: 30 MARCH 2011
Welcome to Volume 10, Issue #6 (30 MARCH 2011) of Pharma Marketing News.
Pharma TeleWeb e-Detailing
This article summarizes Eli Lilly's European experience using TeleWeb e-detailing, which combines a personal sales rep phone call with Web site surfing.
Pharma TeleWeb e-Detailing
This article summarizes Eli Lilly's European experience using TeleWeb e-detailing, which combines a personal sales rep phone call with Web site surfing.
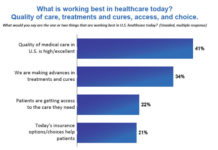
Are Sales Reps as “Useful” as PhRMA Wants Us to Believe?
Let's Look at Some Data PhRMA Doesn't Mention. PhRMA pointed out that nearly 9 out of 10 physicians considered company-sponsored peer education programs to be 'up-to-date, useful and reliable.'
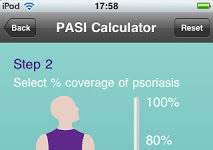
FDA Issues Long-Awaited Guidance for Mobile Medical Apps
The Center for Devices and Radiological Health (CDRH) and the Center for Biologics Evaluation and Research (CBER); responsible for regulating medical devices, have issued guidance for mobile medical applications. The guidance focuses only on a select group of applications. Meanwhile, many pharmaceutical companies, such as Janssen, are developing mobile apps for the iPhone and iPad. Can some of these apps be considered medical devices requiring approval by the FDA?



![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)



