Delving Deeper Into Drug Risks The need for pharma to demonstrate the value of their products for driving optimal improvement in public healthClick Here for Additional Resources
A conversation with John J. Doyle (see bio), Sr. Vice President and Managing Director – Global Market Access at Quintiles, about the need for deeper knowledge of the inherent risks of pharmaceutical products. This need was revealed by The New Health Report 2012, which is based on surveys of biopharmaceutical executives, managed care executives in the U.S., National Health Service executives in the U.K., patients living with chronic disease in both the U.S. and the U.K., and investors who focus on the healthcare sectors.
Aired LIVE on: Friday, June 15, 2012 Listen to internet radio with Pharmaguy on Blog Talk Radio
You can also listen here: Pharmaguy BlogTalk Radio Page. This show and ALL Pharma Marketing Talk shows are available as podcasts via PMT on iTunes (FREE!).
BackgroundThe essence of healthcare arises from the tradeoffs between risk and value. Last year’s New Health Report focused on value — patients, physicians, payers and biopharmaceutical executives defined this concept in many ways. For example, only two percent of patients polled mentioned cost and outcomes when defining value as opposed to 38 percent of biopharmaceutical executives. As with value in healthcare, risk extends across the constellation of stakeholders, and each group sees risk from a unique perspective.
Given the global macro-economic environment and many governments’ focus on balancing unfeasible budgets through austerity programs, the pressure for biopharmaceutical companies to demonstrate the value of their products for driving optimal improvement in public health has never been greater. As such, resulting policies — i.e., reimbursement and coverage decisions — will in large part be dictated by a framework that includes a formulaic process for determining a biopharmaceutical product’s value relative to its risks.
Yet, while centralized paying bodies are a critical component for the ultimate commercial success of a new treatment, all other stakeholders within the healthcare ecosystem — biopharmaceutical companies, providers, patients and financial investors — also must determine the tradeoffs of how much of value is worth being put at risk, without the benefit of having either a standardized or centralized formula for doing so.
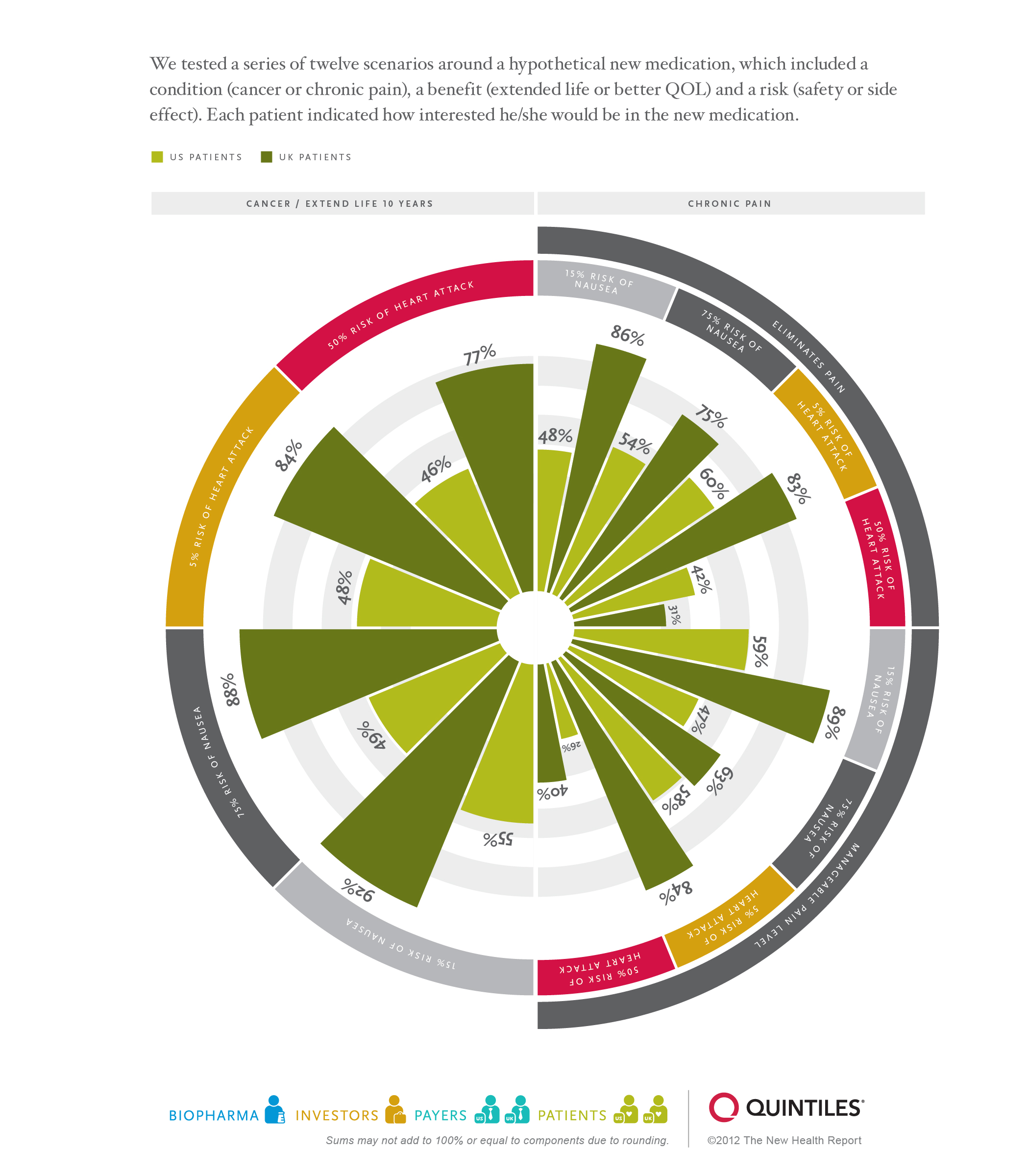
Click here for larger view.
Questions/Topics To Discussed
- Tell us the methodology of the surveys that are analyzed in this report.
- Please review for us the key findings of the report.
- Why is there such a big diff between US and UK patients? Does it have something to do with lack of DTC advertising in UK (i.e., US DTC have conditioned US consumers to think of drugs as more risky than their UK counterparts who have not been exposed to daily messages about risks)?
Guest Bios
 John J. Doyle
John J. Doyle
Dr. John Doyle leads the Global Market Access practice for Quintiles Consulting, focusing on helping life sciences companies maximize the commercial success of their products through market access strategy and evidence-based research. Consulting service areas include pricing and reimbursement strategy, health economic and outcomes research evaluations, health technology assessments, and scenario planning.
Previously John served as President of Analytica International, a bio-pharmaceutical consultancy based in New York, NY and Lörrach, Germany specializing in market access strategy. Prior to that, John headed the Oncology and Immunology Economics Research Group at Bristol-Myers Squibb Company.
Dr. Doyle is on faculty in the Departments of Epidemiology and Healthcare Policy & Management at Columbia University’s Mailman School of Public Health, where he teaches courses in pharmacoeconomics and pharmacoepidemiology.
Additional Resources









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




