FDA’s User-Unfriendly Adverse Event Reporting System: Outdated in the New Transparent World of Social Media A conversation with Brian M. Overstreet (see bio), founder and president of Adverse Events, Inc., about the challenges FDA faces in reporting adverse drug events to the public and the impact on patient safety.
Aired LIVE on:
Friday, September 16, 2011 Listen to internet radio with Pharmaguy on Blog Talk Radio
You may also visit this Pharma Marketing Talk Segment Page to listen to the audio podcast.
This show and ALL Pharma Marketing Talk shows are available as podcasts via PMT on iTunes (FREE!).
[NOTE: The chart shown here is the results of “Harry’s Drug Risk Parlor Game”, a survey that aks respondents to rate the risk Rx drugs in gemeral. Take the survey here and see the results to date.]
Background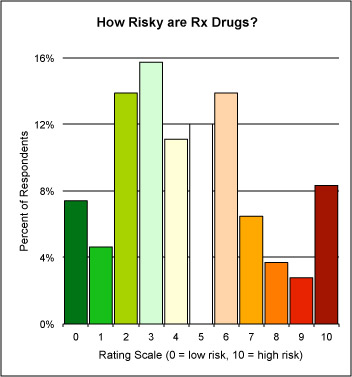 Most data regarding drug side effects resides on the Food & Drug Administration’s Adverse Event Reporting System (AERS), a computerized information database designed to support the FDA’s drug safety surveillance program for its approved drugs.
Most data regarding drug side effects resides on the Food & Drug Administration’s Adverse Event Reporting System (AERS), a computerized information database designed to support the FDA’s drug safety surveillance program for its approved drugs.
“I can’t fault the FDA for its efforts to collect the information and make it available; it’s a massive undertaking,” said Mr. Overstreet. “But the database is poorly organized and almost impossible to navigate, especially for consumers.”
The FDA has traditionally moved slowly to increase public access to relevant drug side effect information. However, there has been a recent shift to change all of this as more and more patients are no longer waiting for the FDA to take action and are taking matters into their own hands.
This has shaken up the world of pharma, and has perhaps been best highlighted by Facebook’s recent unprecedented move to align itself with patients to increase transparency on drug company Facebook walls, making all comments public in regards to medication concerns and side effects leaving drug manufacturers at a crossroads, in contrast with the transparent world of social media.
Drug manufacturers now face challenges in managing their messaging, particularly in the social media realm, as consumers continue to demand increased transparency and accessible, accurate information about side effects and several patient tools are on the horizon, increasing access to medication and side effect information in light of the inefficient FDA process in testing, approving, and monitoring of prescription medications.
Click Here for Additional Resources
- What are the current challenges within the FDA medication database?
- How do they effect patient safety, and how is AdverseEvents looking to solve this problem?
- As AdverseEvents achieves this goal and drug side effect information becomes more available, what might the implications for pharmaceutical marketing be? Perhaps you can discuss the specific case of Gardasil, which has been in the news recentlty (Michele Bachmann claims that a young girl was made mentally retarded by injections of the Gardasil vaccine). Merck lobbied hard to make Gardasil vaccination mandatory in schools and Texas Republican governor Richard Perry complied, signing an executive order back in 2007.
Guest Bio
 Brian Overstreet manages Adverse Events, Inc.’s strategy and operations. Mr. Overstreet brings more than fifteen years of experience building and managing companies in the data, research and investment industries.
Brian Overstreet manages Adverse Events, Inc.’s strategy and operations. Mr. Overstreet brings more than fifteen years of experience building and managing companies in the data, research and investment industries.
Prior to joining AEI, Mr. Overstreet co-founded Sagient Research Systems, Inc., publisher of specialized research and data, where he served as president and CEO of the company for ten years, and currently serves as Chairman of the Board. While at Sagient Research, Mr. Overstreet was responsible for the development, production and sale of the Company’s proprietary research products to global enterprises including pharmaceutical companies, investment banks, mutual and hedge funds, academic institutions and government agencies.
More recently, Mr. Overstreet co-founded Bruliam Wines with his wife, Dr. Kerith Overstreet. As a boutique California winery that has earned a number of 90+ point scores from Wine Enthusiast magazine, Bruliam Wines donate 100 percent of all profits to charity.
Mr. Overstreet earned a B.A. in Political Science from the University of California at Berkeley.
Additional Resources
- Adverse Events, Inc. web site
- How Risky are Rx Drugs?
- FDA Widgets: How About One for Adverse Event Reporting?
- PatientsLikeMe Reports High Rate of Adverse Event Reporting Among Its Members
- Twitter for Patient Support: Not as It Is, but as It Could Be
- Twitter and Drug Safety: A Paramount Concern
- FDA Considers Color Code for Food Labels, But Not for Drug Labels
- Gardasil: To Be Mandatory or Not To Be Mandatory — That is the Question









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




