REMS Impact on Pharma Marketing Survey
Survey began 10 August 2009
This survey solicits your opinion on the impact of REMS on pharma marketing.
See Resources & Further Reading below…Background Risk management. REMS (Risk Evaluation and Mitigation Strategy). Danger management of your product’s life cycle.
These topics are all crucial to brand managers and marketing executives. Did you know that a Risk Evaluation and Mitigation Strategy (REMS) can be a win-win-win situation for you, your product, and the patient? Or that the longer you wait in the product development process to start thinking about REMS, the more you will lose control of your product’s future?
A Risk Evaluation and Mitigation Strategy (REMS) is a strategy to manage a known or potential serious risk associated with a drug or biological product. A REMS will be required if FDA finds that a REMS is necessary to ensure that the benefits of the drug or biological product outweigh the risks of the product, and FDA notifies the sponsor. A REMS can include a Medication Guide, Patient Package Insert, a communication plan, elements to assure safe use, and an implementation system, and must include a timetable for assessment of the REMS.
Survey questions include:
- How useful do you think the following could be in improving physician understanding of the benefits and risks of a new drug coming to market?
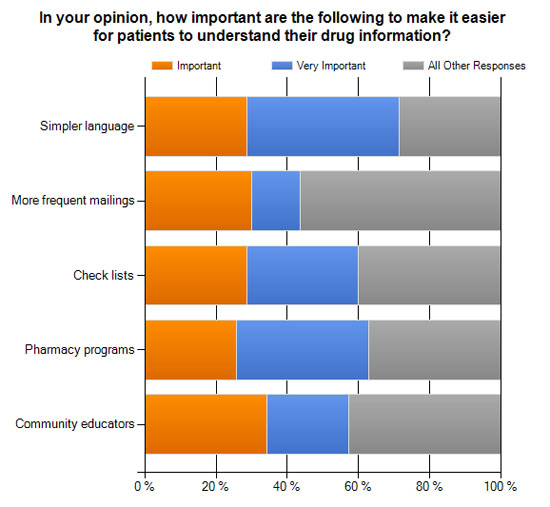
- In your opinion, how important are the following to make it easier for patients to understand their drug information? (see chart below)
- After product launch, what role do you think marketing should play in assessing REMS effectiveness?
- In your future Direct-to-Consumer marketing, how important will REMS be in changing your marketing strategy?
- In light of REMS, how often do you plan to use the following communication channels in your marketing strategy?
- Others…
Take the Survey
Please take 2 minutes to answer this survey relating to Impact of REMS on pharma marketing. Take the survey here.
You will be able to see a summary of up-to-date de-identified results upon completion of the survey.
Results of this survey may be summarized in an issue of Pharma Marketing News.
Your comments are confidential (anonymous) unless you specifically provide your contact information at the end of the survey and allow us to attribute comments to you personally.




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




