Direct-to-Consumer Off-Label Drug Promotion Survey
Survey began 4 September 2016
Survey will end 7 November 2016
Background
Under pressure from the drug industry and “First Amendment” legal issues, the FDA has hinted in a recent public hearing notice that it may allow pharmaceutical and Medical device companies to engage in off-label communications directly to “patients and consumer audiences” and not just to physicians.
In the Federal Register Notice [Docket-FDA-2016-N-1149] announcing the public hearing (“Manufacturer Communications Regarding Unapproved Uses of Approved or Cleared Medical Products”), FDA asked these questions related to the off-label promotion of drugs and medical devices to consumers:
“To what extent is it appropriate for firms to communicate information about unapproved uses of their approved/cleared medical products to patient and consumer audiences? What disclosures and additional information would be needed to help ensure that a communication to lay audiences is truthful and non-misleading, given consumers’ lack of medical training and expertise in critically evaluating this type of information?”
For more background, read “FDA May Have No Choice But to Allow Direct-to-Consumer Off-Label Drug Promotion”
Preliminary Results
The following preliminary results from the survey were submitted as comments to Docket-FDA-2016-N-1149 on 14-Oct-2016 (Comment Tracking Number: 1k0-8sge-hi48; download PDF). A more complete summary will be presented at the Public Hearing on November 7, 2016. Your de-identified responses and comments will be included.
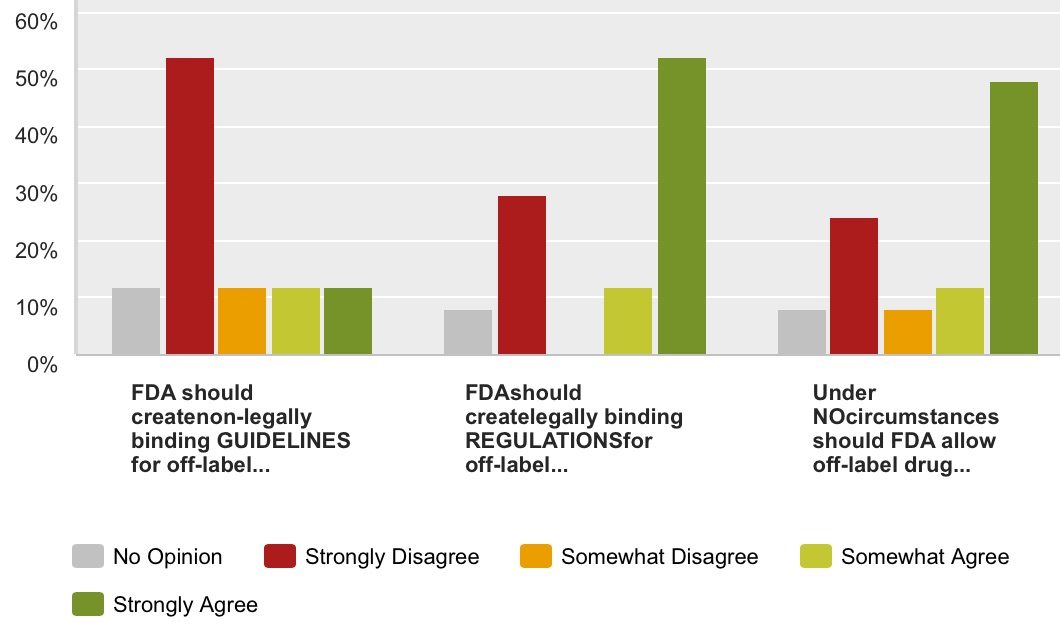
Q1: Indicate your level of agreement/ disagreement with the following statements. Note: FDA regulations are legally-binding federal laws, whereas guidelines have no legal standing. “Promotions” include print and TV advertising as well as drug.com websites and other forms of digital advertising.
- FDA should create non-legally binding GUIDELINES for off-label direct-to-consumer promotions by pharma companies.
- FDA should create legally binding REGULATIONS for off-label direct-to-consumer promotions by pharma companies.
- Under NO circumstances should FDA allow off-label drug promotion to patients or consumers.
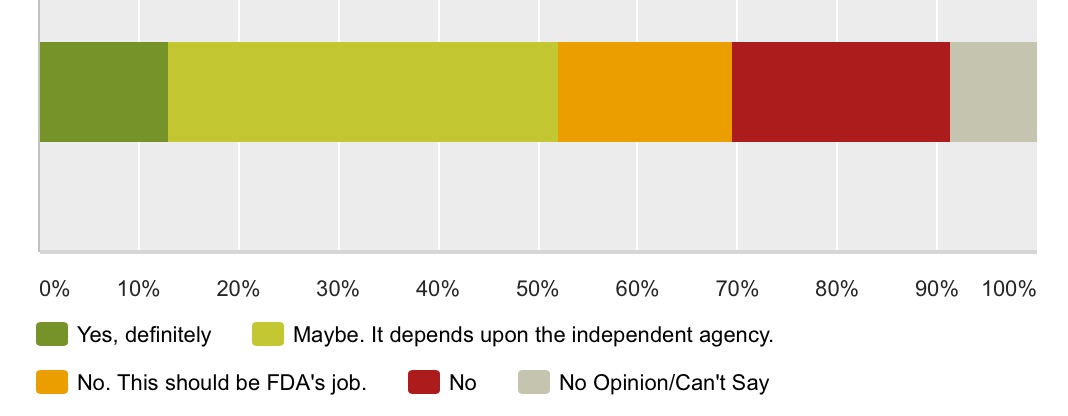
Q2: A Duke University think tank suggested that a new independent entity be created to review claims and recommend exactly what off-label information drug and device makers should be allowed to share with doctors. Should such an independent entity do the same with regard to off-label information shared with consumers?
Please Take the Survey 
Direct-to-Consumer Off-Label Drug Promotion Survey This survey solicits your opinion possible FDA future decisions regarding off-label drug promotion to patient and consumer audiences. For more background information, read “FDA May Have No Choice But to Allow Direct-to-Consumer Off-Label Drug Promotion.“
CLICK HERE to take the survey.
Your comments are confidential (anonymous) unless you specifically provide your contact information at the end of the survey and allow us to attribute comments to you personally.




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




