Should FDA Be Reformed?
(Survey started 20 December 2004; ended 15 February 2005; N = 39)
 In the wake of the VIOXX withdrawal, critics have suggested that FDA needs to reform how it regulates the drug industry. Many suggestions for reform have been suggested. This survey tested the viability of a few of these suggestions.
In the wake of the VIOXX withdrawal, critics have suggested that FDA needs to reform how it regulates the drug industry. Many suggestions for reform have been suggested. This survey tested the viability of a few of these suggestions.
See Summary of Results and Resources & Further Reading below…
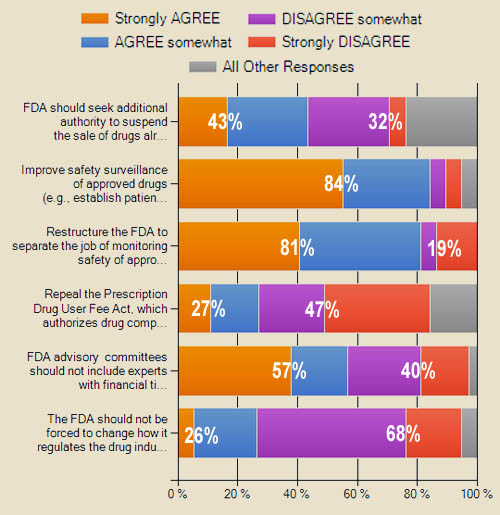
Partial results are summarized in chart below. Access a more detailed and up-to-date online Summary of Responses plus view comments from respondents here.
Respondents were asked to indicate how strongly they agreed or disagreed with several suggestions for reforming the FDA put forth by various stakeholders. Suggestions:
- “FDA should seek additional authority to suspend the sale of drugs already on the market”
- “Improve safety surveillance of approved drugs (e.g., establish patient registries and/or require drug companies to undertake new safety tests once a drug is approved)”
- “The FDA should not be forced to change how it regulates the drug industry. It is doing a good job right now.”
- “FDA advisory committees should not include experts with financial ties to the drug industry”
- “Repeal the Prescription Drug User Fee Act, which authorizes drug companies to pay fees to FDA for each drug reviewed”
- “Restructure the FDA to separate the job of monitoring safety of approved drugs from the job of approving drugs in the first place”
- Pharma Marketing News article: Does the FDA Need to be Overhauled?




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




