The Value of Direct-to-Consumer Advertising
(Survey started 28 September 2005; halted 8 January 2006, re-opened 15 November 2011. N=32 respondents as of 15-Nov-2011)
![]() On November 1-2, 2005, the Food and Drug Administration (FDA) hosted a 2-day public hearing to provide an opportunity for broad public participation and comment on direct-to-consumer (DTC) promotion of regulated medical products, including prescription drugs for humans and animals, vaccines, blood products, and medical devices.
On November 1-2, 2005, the Food and Drug Administration (FDA) hosted a 2-day public hearing to provide an opportunity for broad public participation and comment on direct-to-consumer (DTC) promotion of regulated medical products, including prescription drugs for humans and animals, vaccines, blood products, and medical devices.
This survey collects comments regarding DTC issues discussed at a November 2005 FDA public hearing. These issues relating to the value of DTC advertising for consumers and pateints are as pertinent today as they were in 2005. Click here to take the survey and see the latest results when you finish.
See Summary of Results and Resources & Further Reading below…
FDA was particularly interested in hearing the views of individuals and groups most affected by DTC promotion, including consumers, patients, caretakers, health professionals (physicians, physicians’ assistants, dentists, nurses, pharmacists, veterinarians, and veterinarian technicians), managed care organizations, and insurers, as well as the regulated industry. FDA sought input on a number of specific questions, but heard other pertinent information from participants in the hearing.
Regarding the educational merits of DTC advertising, FDA wanted to know if DTC needs additional educational content about the disease or condition. “FDA is … specifically interested in whether paying greater attention to the educational component of an advertisement (i.e., devoting more attention to defining the disease and its manifestations) would help consumers better understand the role drug and device therapy may play in treating that disease.”
Regarding the presentation of risk information in DTC ads, the FDA “is interested in hearing why consumers and healthcare providers may believe that risk information is not being communicated as clearly as benefit information, even though that information is present.”
Partial results are summarized in charts below. You can view a more detailed, complete and up-to-date online Summary of Responses plus view comments from respondents after taking the survey yourself: here.
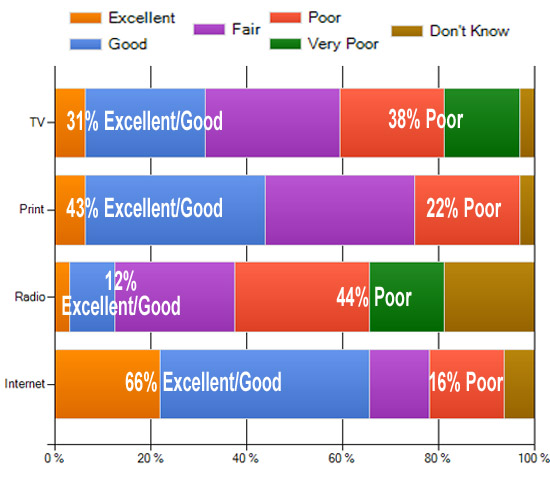
Disease Education
Respondents were asked “How good a job are pharma marketers currently doing to educate consumers about diseases in DTC advertisements in the following media (TV, Print, Radio, Internet). Results to date are shown below. What’s your view? Take the survey and tell us (you can view up-to-date survey results plus comments after taking the survey here).
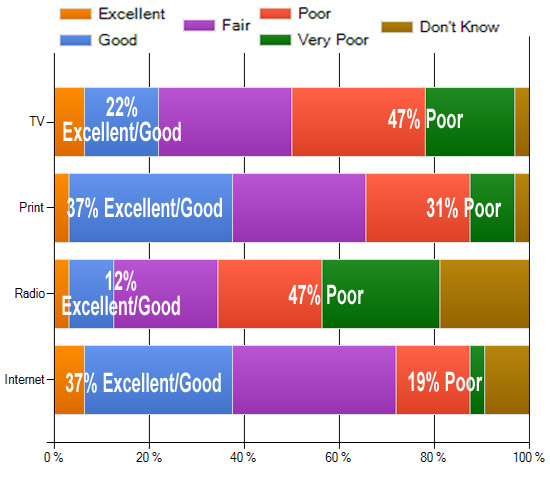
Communicating Risk
Respondents were asked “How good a job are pharma marketers currently doing to communicate drug risks in DTC advertisements in the following media? (Consider if risk information is presented in clear, understandable language, without distraction from the content, and in a manner that supports the responsible dialogue between patients and health care professionals.)” (TV, Print, Radio, Internet). Results to date are shown below. What’s your view? Take the survey and tell us (you can view up-to-date survey results plus comments after taking the survey here).
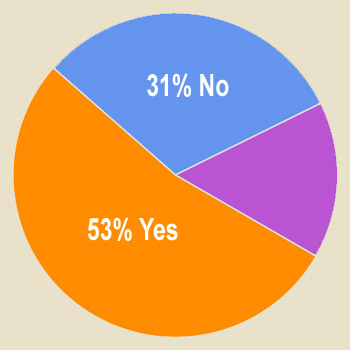
Use of Celebrities
Another standard marketing technique uses real people, or actors portrayed as real people, to provide positive reports (testimonials) about an advertised product. Applied to medical products, this technique portrays patients who describe how a drug or device helped them manage their medical condition. In rarer instances, healthcare providers, or actors portraying them, vouch for the use of the product. Such approaches, says FDA, plainly do not reflect a data-oriented approach to promotion and may not be recognized by consumers as anecdotes.
Respondents were asked “In your opinion, does the use of celebrity — or real patient — endorsements or actors playing doctors in DTC ads mislead consumers about the risk-benefit tradeoffs of prescription drugs?” (Answer: Yes, No, or No Opinion). Results to date are shown below. What’s your view? Take the survey and tell us (you can view up-to-date survey results plus comments after taking the survey here).
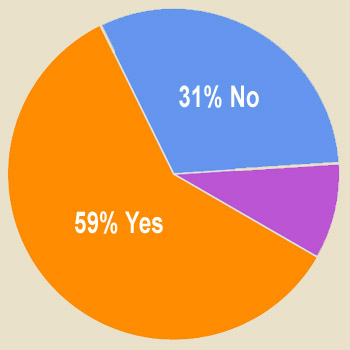
In 2006, Pfizer pledged to “invest a meaningful amount — on par with what it spends on a branded advertisement campaign” to create non-branded ads such as disease-awareness ads and compliance ads. Respondents were asked “Should the pharma industry as a whole adopt this policy and make it a permanent policy?” (Answer: Yes, No, or No Opinion). Results to date are shown below. What’s your view? Take the survey and tell us (you can view up-to-date survey results plus comments after taking the survey here).
- Article: “DTC Pros and Cons Presented at FDA Hearing
- Pharma Marketing Blog Post: “FDA May Follow PhRMA’s Lead on DTC
- FDA: “Results from FDA Physician Survey on DTC Advertising“




![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




