John LaMattina, former Pfizer president of Research and Development, suggests that FDA regulations make it virtually impossible for the pharmaceutical industry to “hide” drug side effects. “Most people don’t have a clue as to the vigorous requirements put on pharma by the FDA for reporting a drug’s side effects,” says LaMittina. “Actually, specific regulations exist as to how the industry must handle and report adverse events” (see “Can Pharma Hide Side Effects Of Marketed Drugs In The U.S.?“).
LaMattina laments that he “often get[s] attacked that I am defending an evil empire.” Some might say a “criminal organization” (see “GSK, GSK, GSK: TSK, TSK, TSK!“).
I don’t want to “attack” LaMattina, but I will point out one or two things that bother me about his post.
First, he insults the intelligence of “most people” who he claims “do not have a clue” about the constraints under which the drug industry operates and which REQUIRE that drug companies report adverse events. The tone, IMHO, is a bit paternalistic and echoes the attitude I’ve heard expressed by other current or former drug industry executives. See, for example, “Bayer’s CEO Accuses Patients of Being Ungrateful B*stards! We Cured Cancer, Dammit!“
Up until now, the main way that healthcare professionals and consumers report drug adverse events (AEs) is through the voluntary Medwatch program, which LaMattina himself says is “not perfect.” That’s an understatement. In 2011, the Tufts Center for the Study of Drug Development gathered and analyzed 10.2 million adverse event reports filed with the MedWatch system. “Patient information was generally complete and accurate. Suspect product information, on the other hand, showed high levels of incomplete and inaccurate data” (see “Evaluating the Completeness and Accuracy of MedWatch Data“).
Also according to PatientsLikeMe (PLM) CEO James Allen Heywood, MedWatch collects only a small percentage of total AEs. He also said: “MedWatch is hostile … and written in a language that patients don’t understand.”
Yes, most people have not heard of MedWatch. Even if they did, however, the system would not capture most adverse events.
So, there really is no need for drug companies to actively “hide” adverse events — they just need to keep the status quo and the system will do it for them.
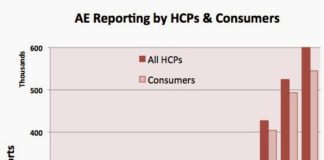
But social media threatens to upset the status quo as far as adverse event reporting goes. PLM found that 7% of 500 randomly selected posts from the 364,000 posts contributed by patients within the PatientsLikeMe Forum during 2009 incorporated all four elements required for reporting an adverse event (an identifiable patient, a specific medication, an identifiable reporter and a reaction). (See “PatientsLikeMe Reports High Rate of Adverse Event Reporting Among Its Members“.)
Recently, I interviewed Pharma Social Media Pioneer Alex Butler who pointed out that pharma may face an adverse event crisis amplified by social media. “We will see very quickly if people are responding badly to medication,” said Alex. “There is bound to be at some point a crisis situation which now in the social world that we live in will be amplified beyond people’s imagination and the impact it will have, possibly with some drug withdrawal, some situation that’s mismanaged” (listen to a snippet from my interview here).
If the drug industry is REALLY interested in NOT hiding the side effects of drugs, then I suggest it follow the advice I gave back in September, 2009 (here); i.e., each drug.com website should include an AE reporting widget as in this mockup:
The widget would include patient education about adverse events and how to use the Medwatch system to report events, using easy-to-understand language and an online Medwatch form that pipes reports directly to the FDA.
If the industry has nothing to hide, it should use technology to help patients report adverse events. Or the industry can maintain the status quo until Butler’s worst case future AE scenario unfolds.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)