 Long before there was a Hanna Montana there was a Wanda Nevada — a 1979 movie starring Brooke Shields and Peter Fonda. I saw the movie yesterday.
Long before there was a Hanna Montana there was a Wanda Nevada — a 1979 movie starring Brooke Shields and Peter Fonda. I saw the movie yesterday.
I also saw the first TV ad for Latisse starring Brooke Shields last night during a re-run of “Desperate Housewives.” Latisse is Allergan’s “new” drug approved by the FDA for “hypotrichosis.”
Hypotrichosis, according to a Wikipedia entry, “is the term dermatologists use to describe a condition of no hair growth. Unlike alopecia, which describes hair loss where formerly there was hair growth, hypotrichosis describes a situation where there wasn’t any hair growth in the first place.”
That, however, is NOT how Allergan describes the condition. According to the “Patient Information” sheet, “Hypotrichosis is another name for having inadequate or not enough eyelashes.”
I imagine asking asking a woman if she has “adequate” or “enough” eyelashes is like asking a man if he has a “big” enough or “hard” enough penis. It is unlikely, therefore, that any woman wouldn’t want, at least, to try this product once.
BTW, the “full prescribing information,” which I am sure nary a single consumer will ever read, says “LATISSE™ (bimatoprost ophthalmic solution) 0.03% is indicated to treat hypotrichosis of the eyelashes by increasing their growth including length, thickness and darkness.” Nothing about “inadequacy” or “not enough” here.
In the TV ad and Latisse Web site, Brooke explains why she was a good candidate for Latisse:
“I thought I’d be a good candidate for Latisse,” says Brooke in her video diary on the Web site, “simply because over the years I’ve just been ripping off my false eyelashes while on Broadway and Allergan approached me and said ‘this is a product, it works, it’s FDA approved,’ and my interest just piqued!”
I’m sure it “piqued” (or should I say “peaked”) even more when Allergan told Brooke how much they were willing to pay her to shill their product! Watch her interest “peak” in the video here. You can also amuse yourself in “Brooke’s Gallery,” where you can see “before” and “after” photos — over a 16-week period — from every possible angle and zoom in and out! From the looks of it, Brooke earned every penny Allergan paid her! I wonder if the product manager got a personalized and autographed photo?
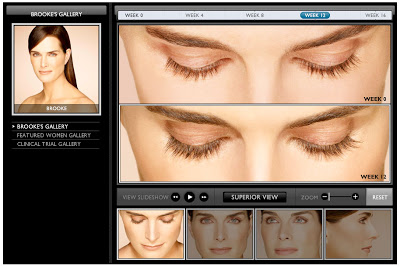 Brooke, like many users of Rx medications before her, is impressed that her medication is “FDA approved.” As if that is equivalent to saying it is completely safe.
Brooke, like many users of Rx medications before her, is impressed that her medication is “FDA approved.” As if that is equivalent to saying it is completely safe.
Recently, the FDA came out with some draft guidance (see “Communicating Risk in Online Drug Ads: Reading the Tea Leaves in Recent FDA Draft Guidance“) that indicates it may have a problem with patients underestimating the side effects of drugs just because FDA has approved them or approved the ads (or statements from drug companies) about them. “Consumers have preconceived ideas about the amount of scrutiny these ads undergo. Many believe FDA exercises tight regulatory control over the content of these ads and to some extent, believe that all ads have been pre-reviewed prior to airing. As a result, consumers are likely to expect that the most relevant risks have been included in the ad.”
Perhaps Brooke is not aware of one the possible “relevant risk” that’s mentioned in the “About Safety” page of the Latisse web site:
“Increased brown iris pigmentation has occurred when similar medications were instilled directly into the eye to treat elevated intraocular pressure/glaucoma. Although iris pigmentation was not reported in clinical studies with LATISSE™, patients should be advised about the potential for increased brown iris pigmentation which is likely to be permanent.”
I have commented on this possible side effect back in October, 2008: “Allergan’s Secret Plan to Thwart Homeland Security and the FDA Approval Process.“
When Allergan says “similar medications,” it really should have said “this medication” because Latisse is just another name for Lumigan, Allergan’s anti-glacoma drug. Both are (bimatoprost ophthalmic solution) 0.03%.
Of course, application of Latisse is outside the eye, whereas Lumigan is applied to the eye. Yet, accidents happen and some Latisse can get into the eye if not carefully applied.
It would be a shame if Brooke’s blue eyes turned brown.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




