My Twitter pals are all atwitter over FDA’s recent letter to Novartis citing violative content in a Facebook “Share Widget” that Novartis created (see “Implications of FDA’s Warning Letter to Novartis Regarding Facebook Share Widget” for the back story).
At the center of the controversy is “metadata.” If you are a pharmaceutical marketer asking yourself, “What the hell IS metadata?”, then you need to read this because some junior web developer on your team (internal or agency side) may be playing fast and loose with your metadata unbeknown to you and your legal/regulatory people. The Novartis letter is a warning that FDA, however, is looking at metadata.
Metadata is usually “invisible” content inserted within the header of the HTML code that creates a Web page. This includes a “description” of the page or Web site and keywords. Some of this information is used by search engines to find the page and include a description of the page in the natural search result.
When you do a Google search on “Viagra,” for example, you will find a “sponsored” link (ie, paid search ad) like this:
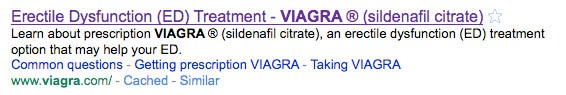
You will also find this unpaid natural search result:
BOTH the paid ad and the search result contain content that is written and controlled by Pfizer. The natural search result content that begins with “Learn about…” is exactly the content that Pfizer included in its “description” meta tag within the HTML code for the viagra.com home page. Google just lifted that content. Users cannot control this content, only Pfizer can — by editing the meta tag.
This was exactly the point that the FDA made in its letter to Novartis regarding the Facebook Share widget. That widget includes meta tag content created and controlled by Novartis. It’s not content that users of the Facebook widget can change.
Let’s look at Viagra.com’s meta tag content. You can easily do this by choosing “Page Source” in the View menu of your browser (eg, Firefox). It may be called something else in other browsers. Here’s what you can find in the source within the “description” meta tag:
“Learn about prescription VIAGRA ® (sildenafil citrate), an erectile dysfunction (ED) treatment option that may help your ED.”
This content is exactly the same content you see in the natural search result above.
The problem is that this content is in violation of FDA regulations that require fair balance — ie, presentation of major risk information — whenever a pharma company controlled communication to consumers includes a product trade name and its indication. The meta tag does NOT include this fair balance.
The lack of fair balance was one of the violations that FDA cited in the Novartis letter that concerned the Facebook widget.
Pharma people have often invoked what I will call the “Invisibility Rule” with regard to metadata. According to the “Invisibility Rule,” meta tag content is NOT intended to be visible or read by consumers; its function is to provide information to search engines and improve the visibility of the site in search engines. Consequently, meta tag content should not be regulated by the FDA.
The problem with the “Invisibility Rule” is that metadata is not really invisible to consumers searching the Internet or using social media because both search engines and social media make the metadata visible.
Digitas Health made this recommendation (see previous post):
“Because website metadata is used both by search engines in generating organic search listings and by social media channels, such as the Facebook Share functionality, Digitas Health advocates that all site metadata should be included in internal medical/legal/regulatory review and as part of mandatory FDA submissions.”
While many pharma marketers may claim not to know about metadata and who creates it — it’s “too technical for me” may be the excuse — the metadata content is carefully crafted by someone on the team as is evident in the Viagra.com example. And that content is directed to the consumer; why else begin with the words “Learn about…” and end with the words “…may help your ED.”?
It is more important than ever that the FDA come out with some guidance relating to this issue, which falls under the heading “Regulatory Solutions to Overcoming Space Limitations in Pharma Social Media Communications” (read more about that here).
Without specific guidance, pharma marketers must rely on “received precedent” and the Novartis letter is such a precedent. It clearly warns pharma advertisers that the FDA is looking at metadata content when that content is controlled by the marketer AND becomes visible to consumers as in Facebook Share widgets. It’s only a short step from widget to natural search result as in the Viagra.com example above. BOTH make metadata content visible and render the “Invisibility Rule” defense questionable.
P.S. Back in the day (prior to 2001) when I worked as a consultant helping pharma companies design and build product Web sites, I had a personal experience with a pharma client who attempted to manipulate meta tag content. Specifically, someone on the client side suggested that competitor product trade names be included within the “keyword” tag of their product’s web page in order to “hijack” searches on their competitor’s trade names. That is, if someone searched for “Brand Y,” the search engine would dutifully offer up the client’s site as a result. I pointed out that this may be illegal and certainly was unethical. The scheme was never enacted and I soon found myself out of work as a consultant.
I tell this story for two reasons:
(1) It offers some evidence that as far back as 2001 at least some pharma marketers knew how to craft metadata content to their benefit; and
(2) It demonstrates how difficult it is to point out that the Emperor has no clothes when you are paid by the Emperor.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




