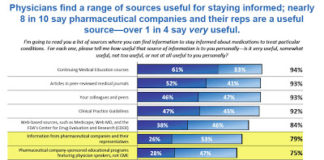
In preparation for FDA’s public hearing on the Promotion of Food and Drug Administration-Regulated Medical Products Using the Internet and Social Media Tools, the agency is asking for comments on 19 specific questions (see “Let’s Respond to FDA’s Questions Regarding Its Regulation of Social Media“). These questions are included in my ongong online survey/questionnaire, which you can access here.
I am following the results of this survey closely and will provide updates. Here, I focus on this question:
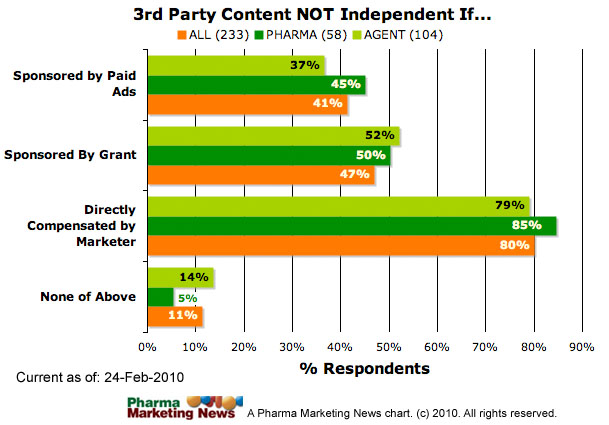
When should third-party discussions be treated as being performed by, or on behalf of, the companies that market the product, as opposed to being performed independent of the influence of the companies marketing the products?
The survey asks respondents to choose one or more of the following responses (and/or add additional comments):
- When marketer or agent sponsors the discussion (eg, provides a specific grant to independent 3rd-party host such as a patient advocacy group to sponsor the discussion)
- When marketer or agent paid for the content (eg, paid patients for testimonials or otherwise provided compensation)
- When marketer or agent paid for display ads to be run on specific discussion pages (eg, only discussions related to the product advertised)
- None of the above
The image below shows how respondents answered this question (ALL respondents vs. PHARMA respondents vs. Agency respondents). The image will be updated frequently. See the date stamp on the image for when it was last updated. To see the most up-to-date results, please take the survey yourself and you will be able to see a summary at the end.
Many comments were submitted in response to this question. Some of these comments include:
- Also, when marketer or agent sponsors a partnership which drives the discussion. For example, if a pharmaceutical company partners with the American Diabetes Association on a program, or an ADA program is made possible because of a grant from industry it should be treated as being performed by the company.
- Unless company is supplying the content or writing script for the discussion, the company is not responsible for the content – merely for any marketing done on or near the content.
- I don’t think companies should be responsible for 3rd party comments in a sidewiki for example.
- The FDA is trying to control a technolgy that moves too fast.
- If the grant is the sole support of the discussion and the patient advocacy, then I would mark that. If it is a grant to patient group and they then use it for a number of programs including the discussion without specifying it as part of the grant application, then no
- Exceptions occur if a grant is provided and marketer has “no say”.
- Agent sponsorship does not have to trigger non-independence if there is full disclosure as is done in scientific journal publications.
- Marketers or agents who take on projects about their company on their own behalf (who are not being supplementally paid for content), should not be tied to FDA regulations. Only those additionally compensated for their social media work should be held responsible for staying in line with industry regulations. However, once ad space is purchased or additional compensation is given, to company employees or agents of the company, there should indeed be stipulations in place to regulate usage.
- When a marketer or agent sponsors the discussion and those making favorable comments to the them are direct or indirect beneficiaries. This would not be apply when who is making the favorable comment is an independent voice
- Marketer or agent built or created the social media site/community tool for the sole use of the company (e.g., marketer or agent builds it own social network for patients vs. sponsoring an existing independent community)
- When marketer or agent controls what is/isn’t posted, i.e. if they screen comments.
- I’m not concerned about display ads, because people know that an ad is a statement by the company.
- If the third-party discussion is initiated by the company that markets the product being discussed, that may trigger non-independence.
- Agree that all three should be treated as being performed by, or on behalf of, the marketer/company, but the first scenario, sponsorship, is less black and white. Should be disclosure for that first scenario, but it’s not as direct line between marketer and content as in scenarios two and three.

SPECIAL REPORT: FDA Regulation of Social Media
Also see:
- What Criteria Determine Substantive Pharma Influence Over Content on Social Media Sites? Survey says…
- Should FDA Regulation Depend on Specific Media or Audiences? Survey says…
- 3rd Party Dissemination of Altered Rx Drug Information on Social Media Sites. Survey says…
- Pharma Companies Should Have Public Social Media Disclosure Policies, Survey Results Show









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)