In preparation for FDA’s public hearing on the Promotion of Food and Drug Administration-Regulated Medical Products Using the Internet and Social Media Tools, the agency is asking for comments on 19 specific questions (see “Let’s Respond to FDA’s Questions Regarding Its Regulation of Social Media“). These questions are included in my ongong online survey/questionnaire, which you can access here.
I am following the results of this survey closely and will provide updates. Here, I focus on this question:
How are entities with postmarketing reporting responsibilities and other stakeholders using the Internet and social media tools with regard to monitoring adverse event information about their products??
The survey asks respondents to choose one or more of the following responses (and/or add additional comments):
- Use of automated keyword searches of selected social media sites by specialized agencies and/or professionals
- Intermittent searches of selected social media sites performed by company personnel or agents
- Intermittent searches of SEARCH ENGINES performed by company personnel or agents
- Routine and automated keyword searches of TWITTER (eg, performed by SocialOomph or other services)
- Use of social media monitoring tools that do not include keywords.
- None of the above
- Don’t Know
Of course, this question is most appropriate to be answered by someone within a pharmaceutical company. Some outside vendors/consultants may also know from their own experience working for pharmaceutical companies.
The image below shows how respondents answered this question (ALL respondents vs. PHARMA respondents vs. Agency respondents).
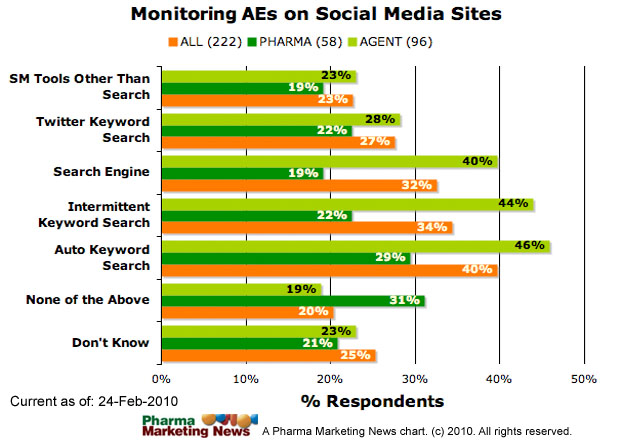
Many comments were submitted in response to this question. Some of these comments include:
- The pharma industry should produce one mass media campaign to inform end users how to get accurate information.
- They should not be responsible for policing these conversations individually. A central industry funded monitoring hub should be established as a clearing house for monitoring public health impact of post marketing issues.
- Adverse events reports about prescription medicines should be made trhough doctors only. For OTC approved medicines different possibiities could be applied
I write for the company blog on adverse reactions, counterfeit drugs, and recalls. - I am not aware of what any companies are actually doing, as opposed to what they say they are doing. However the five options mentioned are worthy of consideration, although I would prefer not to see intermittent searches but rather searches that are conducted on a prescribed routine.
- I do not believe pharma should be required to search for adverse events. For those events where complete information is available, companies must report them when they become aware of the event. FDA should proceed in allowing individuals to file adverse event reports directly to the agency. By doing this, I believe more patients will report events and make the AE reporting more robust. Requiring the companies to chase down every potential report is asking too much.
- Adverse events should be monitored and reported by physicians who prescribe treatments and follow up patients. The physician in contact with the patient should be the filter and the ultimate person who should decide and perform the reporting.
- Currently companies are probably not monitoring social media as much because of the uncertainty about adverse event reporting.
- Have specific site for direct reporting of adverse events. Do not depend on open searches. If something is picked up then direct to adverse event home page.
- I believe entities are using various tools or employing agencies to search what’s being said about their brands, but not necessarily for AER however.

SPECIAL REPORT: FDA Regulation of Social Media
Find links to more results here.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




