The FDA recently posted a new social media policy, but it’s not for the pharmaceutical industry. The policy is for its own employees (read “FDA Releases a Long-Awaited Social Media Policy“).
Like similar policies developed by pharma companies such as Roche (here), FDA’s SM policy provides guidance to their employees about how to use social media for personal and professional purposes.
“The FDA encourages the use of social media technologies to enhance communication, collaboration, and information exchange in support of FDA’s mission to protect and promote public health,” states the introduction. “This policy applies to FDA employees, contractors, and other personnel acting in an official capacity when using social media to communicate with the public regarding FDA-related matters.”
I read “between the lines” to determine if this policy offers any hints as to how the FDA may further regulate pharma’s use of social media in the future, especially with regard to correcting/deleting comments posted to its own sites and linking to external third-party sites.
Let’s look at FDA’s comment policy for its official social media channels – excluding, of course, the FDA Voice blog and FDA Youtube channel, which do not allow comments. FDA’s policy states:
“Each Center/Office will develop a process to administer comments in order to maintain a respectful and on-topic dialogue…Any comment removed by FDA should be recorded and archived prior to deleting.”
So far the FDA has only issued guidance to the drug industry for correcting misinformation on 3rd-party sites, not sites controlled by the industry (see here). However, that guidance did offer a case for how pharma companies can deal with comments posted to its own sites:
“A firm hosts a discussion forum about its drug’s or device’s FDA-approved use on its corporate website and does not participate in the discussion, but it does monitor the forum for profanity and obscenity. The forum includes an overarching clear and conspicuous statement that the firm did not create the content of the forum. The firm is not responsible for the information that is posted by independent third parties and can, it so chooses, correct misinformation according to this guidance.” [my emphasis]
And, when such corrections are made, the guidance states:
“FDA does not expect firms to submit corrections to the Agency when correcting misinformation pursuant to this draft guidance; however, FDA recommends that firms keep records [my emphasis] to assist in responding to questions that may come from the Agency. The records should include, for example, the content of the misinformation, where it appeared, the date it appeared or was located, the corrective information that was provided, and the date the corrective information was provided.”
As noted above, the FDA has not issued guidance to the drug industry specifically regarding correcting misinformation about Rx products that may be posted on sites it owns or for which it is responsible. And it has not issued any guidance about maintaining records for deleted comments.
Will the FDA issue such guidance in the future? And will it resemble FDA’s own policy?
Pharma companies once owned social media sites that included user-genareted discussions about products (see “Question Everything“) and diseases (see “Markets as Conversations: Can You Have a Discussion with “Psoriasis 360” on Facebook?“). These days, however, that would be a rare thing to see. So, FDA may see no need to issue guidance for the industry to correct (or delete) user-generated misinformation posted to its own sites. But, hey, it could still happen!
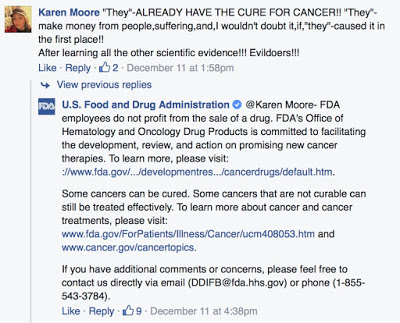
BTW, here’s an example of how FDA corrects”misinformation” on its Facebook page:
This is a “canned” responses, which I have seen in other posts on the agency’s FB page.
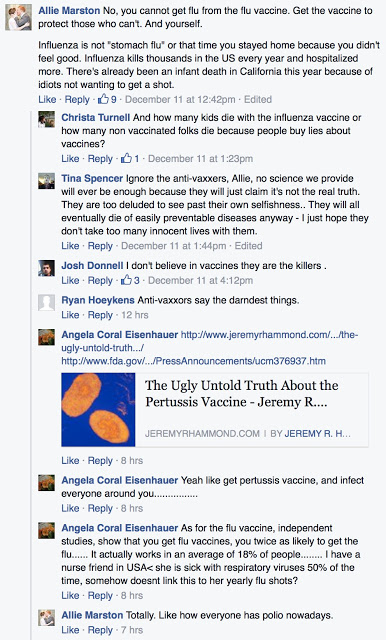
Meanwhile, FDA does not correct other “misinformation” on it FB page, especially when it concerns controversial issues such as vaccination. Here’s an example of a post/discussion that so far has not been responded to by the FDA:
Some replies in defense of vaccines, such as that by Ryan Hoeykens, may be written by FDA employees expressing their own personal opinion. FDA’s SM policy grants them the right to do so:
“Ordinarily,” says the FDA, “an employee need not use a disclaimer to disavow government endorsement of personal social media communications” unless there is a need to correct a possible misimpression that the views expressed are endorsed by the FDA. In that case, the employee “may” use a disclaimer such as “The views and information presented here are mine.” [FDA’s emphasis]
In contrast, the Roche guidelines mentioned above explicitly state that in personal use of social media its employees must “Be transparent about your affiliation with Roche and that opinions raised are your
own.”
What about links?
FDA’s 2014″Guidance Agenda” promised guidelines of “Use of Links,” which was also on the agenda of the November, 2009 public hearing (see “FDA Has One More Internet Guidance to Publish in 2014: Links“). FDA asked “When is the use of links appropriate? The agency is interested in any comments about the appropriateness of various techniques regarding the use of links (including between various social media tools) and data or research about whether or not users find these approaches to be misleading.”
Guidelines for use of links by pharma have yet to be released by the FDA. The Agency, however, has guidelines for how its employees should link to 3rd-party sites from official FDA sites:
- FDA “recommends using Go.USA.Gov (http://go.usa.gov) to create short, trustworthy .gov URLs to use on online services with character restrictions. At this time, Hootsuite (https://hootsuite.com) is also an approved service for creating shortened URLs.”
- “FDA is able to link to websites outside of FDA.gov according to federal linking policies. Links to outside supporting material may be suitable in some instances but in most cases, the FDA should be the authoritative source. Consider FDA’s regulatory mission when using links to any websites outside of FDA.gov.”
Would the anticipated future FDA link guidelines for the drug industry suggest that outside links should be to “authoritative sources?”
One other issue FDA addressed is the right of its scientists to “request” the correction of errors about their work and views published by the FDA on social media channels:
“an agency employee can request that an agency social media communication be corrected, amended, or clarified if
- the communication is based upon the research or published work of the employee or purports to express the employees views by name or title; and
- the communication is false, misleading, or confusing.”
Whether or not FDA’s social media policy is a preview of future industry guidance is a moot point. More importantly, all pharma companies should follow FDA’s lead; i.e., (1) every pharma company should have a social media policy and should make that policy public just as the FDA has done; and (2) pharma company employees — especially scientists — should have the same social media rights as do the employees of FDA.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




