Remember the 14 Notice of Violation (NOV) letters that FDA sent to 14 drug companies citing their Adwords — paid search engine ads — for violating FDA regulations? At issue was (and still is) the so-called “One-Click Rule,” which states that an online Rx drug ad can mention the brand name and the benefits (indications) without including all or any of the major side effect effects (fair balance) as long as the fair balance is just one click away (ie, on the landing page). See “The ‘One-Click Rule’: Rant or No Rant?” and “Death of the One-Click ‘Rule’ or ‘Received Precedent’ or Whatever!” for my long-standing criticism of the “One-Click Rule.”
The FDA letters only focused on paid search engine ads where there is a limit of about 70 characters. You can feel some empathy for pharma marketers in that case because of the impossibility of fitting all that fair balance into 70 characters — impossible!
But what about the 140 characters allowed in Twitter posts? Is the FDA looking at that?
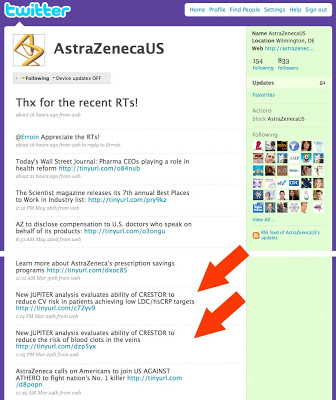
Below is an example of two Twitter posts made by AstraZeneca US on March 29, 2009 — a few days before the 14 letters were sent (BTW, AZ was not one of the companies that received a letter that day).
These Twitter posts (“tweets” include the brand name of the drug (Crestor) and the indications (eg, “reduce CV risk” and “reduce risk of blood clots in the veins”), but not the side effects. Both tweets link to AZ press releases that contain all the risk information.
BTW, you’ll also find links to these press releases on the AstraZeneca (US) web site here. These links also mention the brand name and benefit without mentioning side effects.
This raises a couple of interesting questions:
- Are Twitter posts considered promotions that are subject to FDA regulations?
- What about links to press releases on drug company web sites?
I guess we’ll have to wait for FDA to issue more letters before we can learn what its thinking is on this!








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)