The effort to get the FDA to hold a public hearing on the use of the Internet and social media for the promotion of drugs and medical products has moved onto a new stage: Lobbying legislators via Twitter (called “twilobbying”).
“John Mack’s Saturday blog post, http://pharma-mkting.com/blog/2009/04/fdas-actions-speak-louder-than-its.html, calls on the FDA to hold a public hearing on the use of the Internet and social media for the promotion of drugs and medical products,” says Krū Research. “This is an excellent and reasonable request. And I’d make one other suggestion, send a tweet to all the Senators and Congressmen and women who are on Twitter. Maybe they ‘get’ social media better than the FDA and will see the sound reason of a request for a public hearing.”
“Get the full list of legislators on Twitter here: http://www.congressional140.com/tweeting.php. Send them this tweet, ‘FDA acts against Pharma on e-marketing, yet offers no guidelines! Please force public hearing. Details: http://tinyurl.com/cwqfza‘”
Survey Results
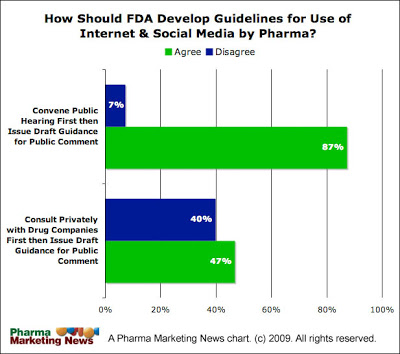
Meanwhile, a survey of nearly 100 pharmaceutical professionals reveals that a vast majority of respondents are in favor of the FDA convening a public hearing before issuing draft guidance and respondents are divided about issuing draft guidance for public review ONLY after consulting privately with pharmaceutical companies (see chart below, click on it for an enlarged view).
Respondents included representatives of the following companies:
- KC Healthcare Communications
- Devine + Powers
- Group DCA
- Cline Davis & Mann
- AMS Inc.
- SMI Health Media
- Thomson Reuters
- Fleishman-Hillard Clinical Trials Division
- Trusted.MD Network
- Good Health Advertising
- Dr. Reddy’s laboratories
- KnowledgePoint360 Group
- HyGro Group, Inc.
- e-Healthcare Solutions
- BioPharma Advisors
- GSK
More details of the survey results will be published in the April 2009 issue of Pharma Marketing News. You can still take the survey and give me your opinions. After you complete the survey, you will be able to see the summary yourself. No comments or other identifying information is included in the summary. Your comments are confidential (anonymous) unless you specifically provide your contact information at the end of the survey and allow us to attribute comments to you personally.
PhRMA Supports a Public Process But Stops Sort of Calling for a Public Hearing
During a recent roundtable discussion (listen to podcast here), Jeff Francer, Assistant General Counsel, PhRMA, said:
“We just want to echo what [Mr. Mack] said, which is it would be helpful for the FDA to have some sort of public process about a guidance document. Without commenting on specific enforcement actions, it’s clear there’s some lack of clarity by companies and it would benefit public health, it would benefit public policy to have a public dialogue especially given the fact that the public is going to the Internet for healthcare information and that there are so many studies that report significant under diagnosis and under treatment of serious diseases in the United States. So that’s why we want to echo your call for a public process.”
PhRMA should issue a public statement and clarify what it means by “some sort of public process.” Usually, the pharmaceutical industry is more comfortable with FDA using a process that includes consultation with the industry and then issuing draft guidance subject to public comment. That, IMHO, is not adequate because the NEW FDA should embrace a more transparent process. A public hearing that is simultaneously streamed live to the Internet would be much more inclusive, transparent, and eduactional.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)