In preparation for FDA’s public hearing on the Promotion of Food and Drug Administration-Regulated Medical Products Using the Internet and Social Media Tools, the agency is asking for comments on 19 specific questions (see “Let’s Respond to FDA’s Questions Regarding Its Regulation of Social Media“). These questions are included in my ongong online survey/questionnaire, which you can access here.
I am following the results of this survey closely and will provide updates. Here, I focus on this question:
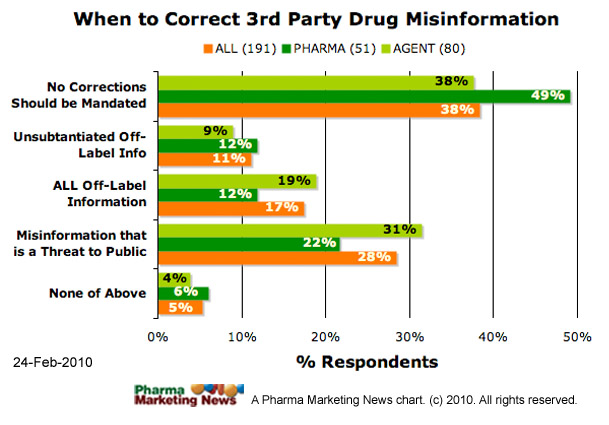
Are there any parameters or criteria that could be used to determine the appropriateness of correcting misinformation and/or scope of information a company can provide when trying to correct misinformation on a Web site outside a company’s control?
The survey asks respondents to choose one or more of the following responses (and add additional comments):
- ONLY misinformation of real and imminent danger to the public health (to be determined by company) should be corrected
- ALL off-label claims — even if supported by peer-reviewed medical literature — should be corrected
- Only off-label claims NOT substantiated by peer-reviewed medical literature should be corrected
- Companies should not be burdened by FDA regulations requiring them to make corrections about ANY product misinformation published on third-party sites
- None of the above
The image below shows how respondents answered this question (ALL respondents vs. PHARMA respondents). The image will be updated frequently. See the date stamp on the image for when it was last updated. To see the most up-to-date results, please take the survey yourself and you will be able to see a summary at the end.
Many comments were submitted in response to this question. Some of these comments include:
- It is in the company’s best interest to correct misinformation but this cannot practically be done in all cases. Too much of a burden to monitor.
- One issue is that manufacturers will have this burden and generic manufacturers will not
- Companies should be permitted to update third-party sites to correct misinformation but not held responsible if subsequent changes re-introduce misinformation.
- Would be nice to correct all the published misinformation on pharmaceutical or health care products… I think it is impossible to find all of them
- Guidance should be given on how companies can navigate this area but it should not be mandatory.
- Who would decide what is of real or imminent danger? Regarding off-label claims supported by peer review, Medicare and many insurance companies accept this info for funding many new drugs, particularly in oncology, so why should a company try to correct this. Finally, it would be difficult for a company to know of all the potential off-label claims and uses that occur every day, several of which occur when drugs are used in combination therapy and it is another drug that is being used off-label, thus making both products’ use off-label. As well, there is every time a different dose is used based on the treating physician’s judgement. It would be impossible to monitor this, let alone take corrective action.
- If you watch what happens when misinformation is posted you’ll see social media channels are quick to highlight misinformation and mistakes. A company or person that puts out misinformation on a regular bases will be dropped by the participants in the network.
- social media should not be considered different from other platforms. Do companies perform corrective action if on their way to work, they pass by a billboard paid for by a third party containing erroneous information about one of their brands? No. They may ignore it, counter it, or litigate it, but almost certainly not take corrective action against it.
- Guidance in this area is needed, and some flexibility based on multiple factors/considerations – including clinical relevance, public health impact, and impact of the misinformation
- off-label use of a product isn’t necessarily bad (e.g. Byetta with TZD at launch was off-label though clinical data was in front of the FDA for the indication). This kind of discussion takes place ALL the time when one HCP solicits the information from another. Who is to define imminent danger? What may be a huge ordeal to one person may not be to another. It seems like a very slippery slope.
- In my opinion pharma companies should have the freedom to correct, but not the responsibility to act as a watchdog. Otherwise that would be an unbearable burden.
If the company is not affiliated with the site in any way, they should not be responsible for the content put out by that site. - They should constantly educate the public that their own website has the appropriate information and comment on their website about inaccuracies seen throughout the web.
- Companies should contribute post approval money to a watchdog government agency that at least reports on Internet misinformation.
- Because of the evolving nature of social media, it would be unrealistic to require companies to monitor and correct misinformation across all social media vehicles. Instead, a standardized approach should be formulated that could be applicable across several types of social media vehicles. the viewer of the information would need to somehow be assured that the communication was coming from a legitimate (company) source, and therefore could be trusted as accurate. If these parameters (at a minimum) could be met, then the manufacturer should retain the right to correct misinformation as they see fit in whichever social media vehicle they choose.

SPECIAL REPORT: FDA Regulation of Social Media
Find links to more preliminary results here.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




