A New England Journal of Medicine (NEJM) Perspective (“Drug Safety in the Digital Age“) suggests that the FDA “update or automatically feed new safety communications to Wikipedia pages.” The authors cite this precedent: “In 2008, the FDA partnered with WebMD to bring public health announcements to all registered users and to quickly integrate this information into WebMD’s suite of Web pages.”
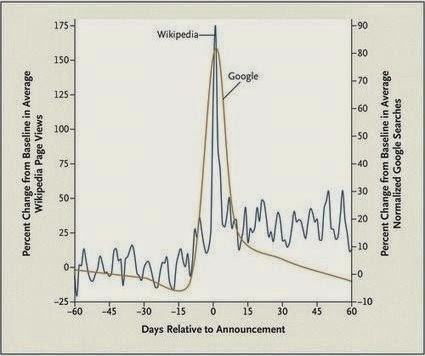
The reason this was suggested is apparent from the following chart, which shows that FDA safety warnings were associated with an “82% increase, on average, in Google searches for the drugs during the week after the announcement and a 175% increase in views of Wikipedia pages for the drugs on the day of the announcement, as compared with baseline trends.”
Given that insight, the authors believe it is imperative that the drug information consumers find on Wikipedia reflect the most recent information viz-a-viz drug warnings from the FDA. The problem is that it takes a long time — if ever — for edits to be made: 36% of the relevant Wikipedia pages remained unchanged more than 1 year after FDA issued warnings.
The authors did NOT suggest that pharmaceutical companies “partner” with Wikipedia to correct/edit drug information on Wikipedia.
Why not?
Drug companies have far greater resources at their disposal to correct information on Wikipedia than does the FDA. And, whenever asked, drug companies are adamant that they have a responsibility to “ensure that consumers get accurate and up-to-date information about their products.”
Perhaps NEJM did not suggest drug companies take some responsibility because it does not want to bite the hand that feeds it; i.e., sponsors it via ads.
In any case, the authors — who seem very knowledgeable about digital health information (otherwise why would they get to write about it in the NEJM?) — must know about Dr. Bertalan Meskó (@Berci) and his open letter to pharma, which urged the pharmaceutical industry to employ Wikipedia editors to “funnel [their] vast resources” to help keep Wikipedia drug articles accurate and up-to-date (read “Here Come the Pharma Wikipedians“).
To date, no pharmaceutical company has volunteered to do what Berci recommended: Appoint someone from within the company as a ‘spokesperson’ in Wikipedia who would perform all edits on behalf of the company.
Now that the FDA has essentially granted pharma’s wishes that any edits it makes to Wikipedia drug articles should NOT be subject to FDA regulations (see “FDA to Pharma: Forget About Tweets! But You Can Host Discussion Forums About Branded Rx Products, Sorta, & Kinda Correct Misinformation“), perhaps industry will take greater responsibility for drug safety in this digital age.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)