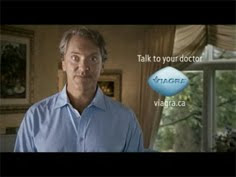
According to Marketing News, Pfizer Canada just launched a 15-second TV ad that promotes Viagra as “an antidote for couples that choose leisurely pastimes over physical intimacy… the spot from Taxi Canada shows a husband confessing that redecorating was ruining their lives: Things like seasonal themes, colour palettes and Feng Shui were getting in the way. ‘So I tried Viagra, and my redecorating practically disappeared,’ he says” (see “Pfizer Canada Launches Latest ‘Confession’ Ad“).
Click here to see the video, then come back to find out why I am confused as to how this ad is allowed in Canada, but “outlawed” in the US.
Pfizer’s “redecorating-vs-sex” Viagra ad is what the FDA and drug industry call a “reminder ad.” According to the FDA, “Reminder advertisements are identified as an exemption to the advertisement regulations, including provisions to provide a brief summary. Reminder advertisements ‘ . . . call attention to the name of the drug product but do not include indications or dosage recommendations for use of the drug product. . . . and, optionally, information . . . containing no representation or suggestion relating to the advertised drug product.’ Reminder advertisements cannot make a representation about the product or suggest a use for the product.” See Pharma Marketing Glossary here for more about reminder ads and sources.
Reminder ads are perfectly “legal” in the US, but PhRMA — the US drug industry trade association — has banned their use in the US by those member companies that have voluntary signed on to comply with PhRMA’s DTC Guidelines. PhRMA DTC Guideline #13 states: “DTC television advertising that identifies a product by name should clearly state the health conditions for which the medicine is approved and the major risks associated with the medicine being advertised.”
I guess only Pfizer (US) is a signatory to the PhRMA DTC Guidelines, not Pfizer (Canada), although I think they are the same company having one CEO. In any case, Pfizer would not be able to run the Canadian ad here in the US.
But that begs the question, why does Pfizer Canada run reminder ads in Canada? Presumably, the DTC Guidelines represent best advertising practices that should be followed in every market, not just in the US.
The guidelines were established because of criticisms that reminder ads had no educational benefit other than placing a brand name in viewers heads. More importantly, reminder ad do not give consumers all the necessary information to make an educated health decision. That, the drug industry claims, is the role of DTC advertising and why it should remain legal in the US. But this whole argument is rendered useless when Pfizer allows itself to air reminder ads outside the US.
So, I say to Pfizer, cease and desist that reminder ad campaign in Canada!
P.S. Did I mention that this Canadian ad is demeaning to women? As if sex would be more important than creative decorating! How sexist is that?








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




