In preparation for FDA’s public hearing on the Promotion of Food and Drug Administration-Regulated Medical Products Using the Internet and Social Media Tools, the agency is asking for comments on 19 specific questions (see “Let’s Respond to FDA’s Questions Regarding Its Regulation of Social Media“). These questions are included in my ongong online survey/questionnaire, which you can access here.
I am following the results of this survey closely and will provide updates. Here, I focus on this question:
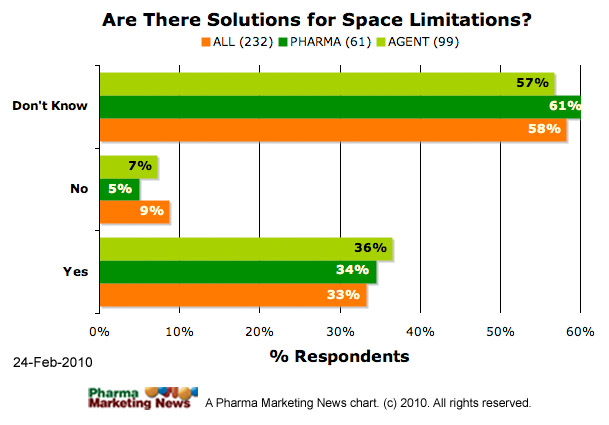
Are there proposed solutions that may help address regulatory concerns when using social media tools associated with space limitations or tools that allow for real-time communications to present product information?
NOTE: FDA has “regulatory concerns” primarily about over emphasis of benefits and/or under emphasis of risks in Rx drug communications by pharmacos.
The survey asks respondents to choose ONLY one of the following responses (and add additional comments):
- Yes
- No
- Don’t Know
The image below shows how respondents answered this question (ALL respondents vs. PHARMA respondents vs. Agency respondents). The image will be updated frequently. See the date stamp on the image for when it was last updated. To see the most up-to-date results, please take the survey yourself and you will be able to see a summary at the end.
Several comments were submitted in response to this question. Some of these comments include:
- The government cannot control communication – see the Bill of Rights
- Very flexible dynamic display or previews of content are possible through most browsers (considering the 2 most recent versions). These provide 1 click access while not navigating a user away from the original content.
- Terms of Use prominently displayed which would include manufacturers guidelines on information
- Mouse-overs to especially call out need to investigate balanced content seems to be a viable alternative.
- Include short URL to safety information: one click away
- Web page banners that provide links to company and government information related to and useful for understanding the topic under discussion.
- I have crafted a set of instructions to address typical FDA regulation and to answer internal regulatory bodies on time and content constraints, though I am not aware of any other companies proposed solutions.
- Companies should have a policy concerning this usage and be responsible for any social media that they or their employees/contractors utilize.
- Technology solutions exist, but don’t know if they have been proposed.
- Phone line indicated in any communication
- Understand in case of microblogging space is limited – disclosures could be on “linked” page.
- People should understand that it’s Twitter… it’s not exactly an exhaustive resource. Links to the reliable information should be sufficient.
- I haven’t seen any proposed solutions. This quiz presents some, such as the one- or two-click rule for disclosure.
- pop ups
- The rule is straightforward. If you present benefits, you must present risks. If the platform is insufficient to present both, alter your tactical use of the platform (i.e. use it as a driver for direct response, make it unbranded, etc.)
- BAn paid Tweets for FDA licensed drug promotional efforts.
- The link to web site information solution noted above

SPECIAL REPORT: FDA Regulation of Social Media
Find links to more preliminary results here.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




