I just learned that Pfizer has developed an iPhone/iPad app that promotes LIPITOR, the drug company’s off-patent lipid-lowering drug. The app is “Recipes 2 Go.” It is the first pharmaceutical app that I know of that promotes a prescription drug.
Here’s what the promo screen on iTunes looks like (click on it for an enlarged view):
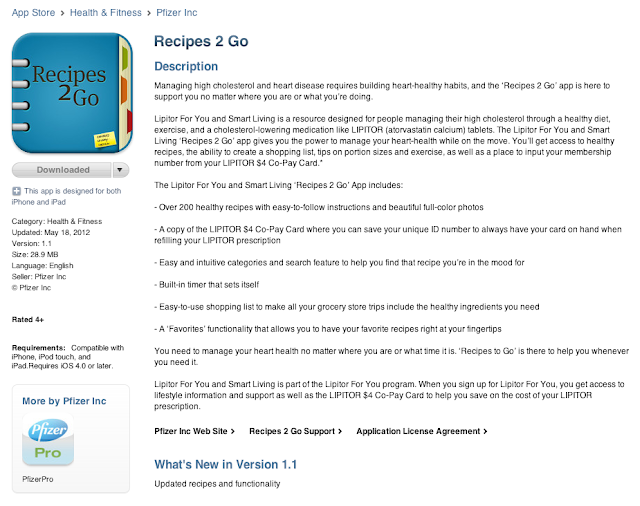
The description — shown here in its entirety — mentions LIPITOR and the FDA-approved indication, which is managing high cholesterol.
Is This an FDA-regulated Drug Ad?
My question: Does this iTunes page qualify as an prescription drug ad that must comply with FDA regulations regarding fair balance (ie, Important Safety Information or ISI)? If it does qualify, then it violates FDA regulations because the page does not include ANY fair balance or a link to the full prescribing information.
Did Pfizer submit this page to FDA for regulatory review? Did it submit the page to its own MLR (medical/legal/regulatory) people?
I downloaded the app to my iPhone and found the side effect/fair balance information plus a link to the “full prescribing” information on the very bottom of the “About Us” screen. Here’s what the screen looks like:
Only when you scroll down to the next screen do you see the notice “Scroll down to see Important Safety Information,” which appears about 14 screens further down! Whew! That’s a lot of scrolling!
The multi-page End-User Agreement (dated April 20, 2012) should be read carefully. For one thing, it states that “NO STATEMENTS MADE IN THIS SOFTWARE HAVE BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION.”
To which I say, Why Not? The FDA should definitely take a look at this app and decide if it complies with regulations.
Because this app does not present the ISI where it can easily be found (and not at all on the iTunes promo page), I gave it only a single star rating on iTunes 🙂









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




