When the drug industry US trade association PhRMA released its Guiding Principles for Direct-to-Consumer (DTC) Advertising in 2005, it was thought that they would affect mostly television DTC ads. Many of the principles apply strictly to TV. However, what effect — if any — has the guidelines had on print DTC ads?
The share of pharma spending on print ads in magazines in the first half of 2006 is up sharply, according to a TNS Media Intelligence study cited in a report by MM&M. Magazines grabbed 34% of total pharma company DTC ad spending during the first half of 2006, up from 29% in the same period in 2005. In comparison, the share allocated to TV DTC decreased from 64% to 59%.
Some analysts contend that print DTC is enjoying a windfall due to the PhRMA guidelines, which became effective in January, 2006.
“Following the adoption of PhRMA’’s voluntary guidelines earlier this year, most companies pledged to improve accuracy and balance in ads. Since this is easier to accomplish in magazine ads, drug makers are using that medium more frequently, TNS research director Jon Swallen told the Associated Press.” (MM&M story)
Principle 11 (Balanced Representation of Benefits and Risks) of the PhRMA Guidelines is the relevant principle here.
“DTC television and print advertising should be designed to achieve a balanced presentation of both the benefits and the risks associated with the advertised prescription medicine. Specifically, risks and safety information in DTC television advertising should be presented in clear, understandable language, without distraction from the content, and in a manner that supports the responsible dialogue between patients and health care professionals.”
You can see that this principle is focused almost exclusively on TV and, at best, is ambivalent with regard to print. Swallen’s point may be that drug advertisers are unsure how to apply this principle to TV ads and are scaling back on TV DTC ads until they can figure it out. Meanwhile, that money has to go somewhere, therefore print ad spending is up.
| Is increased print ad spending due to PhRMA’s guidelines? | |
| Yes | |
| No | |
| Don’t know | |
Following the money, we may need to pay more attention to print DTC than we have in the past. For example, how “balanced” are print DTC ads? If it’s easier to achieve balance in print than in TV ads, then we should see more balance in print, right?
One way to look at this is to measure how much time in TV ads and space in print ads is devoted to risk information.
I have looked at over 60 print ads and measured the ad space is devoted to risk information vs. benefit information in these ads. To get access to the complete dataset, please download the entire September issue of Pharma Marketing News (FREE!).
I haven’t done a study of TV DTC ads, but from what I see and hear, perhaps 20% to 30% (12 to 18 seconds in a 60-second spot) of such ads are devoted to presentation of major risk (fair balance) information.
How do print ads stack up against that?
In my study, print ads I studied that appeared in publications from June through October, 2006, devote only an average of 12% of the creative ad space (not including the “brief summary” page) to risk information.
On that basis, print ads devote even less attention to risk information that do TV ads! Note, however, that I am talking only about the creative area of the ad, not the “brief summary,” which is often the entire drug label that is reproduced on the opposite side of the ad page. Nobody reads this, so I don’t count it.
[About half of the print ads I looked at presented a patient-friendly brief summary page. This Q&A large format style was pioneered by Pfizer. The problem is that even this friendly version is not likely to be read because it appears on the reverse side of the ad page.]
In print ads appearing in publications from February through March, 2004, about 9% of the creative ad space was devoted to risk information.
This tells me two things:
- Print DTC ads devote considerably less ad space to presenting risk information than does TV DTC, and
- Print DTC ads appearing in publications after the PhRMA DTC principles became effective devote more space to risk information (about 33% more) than ads published before the principles became effective.
It appears that print ads rely too much on consumers reading the “brief summary” and therefore less creative ad space is devoted to presenting the major risks. On the other hand, print ads devote an average of 19% of the ad space to benefit statements. If you ad in the graphics, which almost always depict benefits, the total ad space devoted to benefits is 65%.
Do print DTC ads provide enough risk information? It depends. Some brands have more risk potential or potential for more serious risks than other brands and therefore require more space to explain the risks. Some brands, like Botox, are rogues and run only reminder print ads without any risk information at all even though the risks may be devastating.
Among the Top Ten “DTC Spending Champs” in the first half of 2006, here’s the space they devote to risk information in their print DTC ads (sorry, I don’t have data for Ambien/AmbienCR):
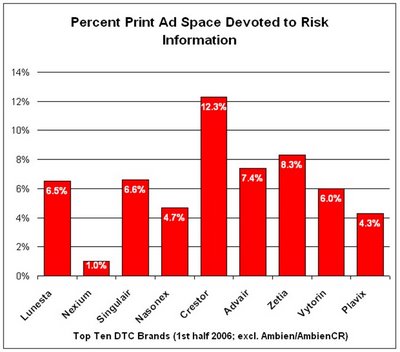 Except for Crestor, all of these top advertised brands devote less than average ad space to risk information in their print ads.
Except for Crestor, all of these top advertised brands devote less than average ad space to risk information in their print ads.
Of course, it’s not just the amount of information that’s important, but the quality of that information. These days, there is a lot of lip service being paid to how to improve the quality of risk information presented in DTC ads. Yet it is very difficult to define “quality.” The FDA is “studying” the issue.
I just don’t believe that presenting “less” risk information in DTC ads as some critics have proposed (see “DTC Without the Risk“) will lead to better quality information.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




