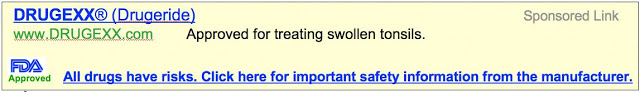
In a telephone news briefing, PhRMA proposed an “FDA-Approved Use of Universal Safety Symbol” that could be used in branded/sponsored ad links (eg, Adwords) and Twitter posts (see image below).
PhRMA says in its slide presentation (see here):
- Universal safety symbol (FDA logo or other FDA-approved symbol) and universal statement would indicate that the linked page contains FDA-regulated risk information (e.g., official Prescribing Information, patient Medication Guide)
- Throughout the Web, a universal symbol would help healthcare professionals and consumers identify official, FDA-regulated medical product Web sites. Prominence of graphic could drive clicks to comprehensive information
- Include established name and true abbreviated indication, if Internet media do not allow for full information
- Include affirmative statement about risks, even if abbreviated
- Universal symbol could be used on search engines, blogs, microblogs, video
- FDA would set conditions on use of the safety symbol by manufacturers
“Leveraging the FDA’s logo – or a universal FDA-approved graphic symbol – in search results and throughout the Web would inform patients, at a glance, that they are visiting a legitimate site that contains comprehensive FDA-regulated benefit and risk information. Such a graphic symbol could be combined with a universal warning statement to provide an indication of risk when there is little space (e.g., a search result or tweet),” said PhRMA (see “PhRMA Statement About Accessing Online Health Information“).
Reporters had several questions about the proposal, including what kind of resources FDA would need to review material before granting use of the symbol and monitoring thereafter. Jeff Francer, Assistant General Counsel at PhRMA, mentioned user fees that PhRMA proposed for FDA review of promotions. “Unfortunately,” said Francer, “congress did not appropriate the money in order for that user fee to go into effect. PhRMA will continue to support a strongly-funded FDA even if it means that user fees from our companies will have to support some of these activities.”
Francer realized that there would have to be some sort of governance structure associated with the use of this symbol, such as that provided by Trustee for privacy policies. “We haven’t gotten into the operational details,” said Francer. “We want to use this as a way to start the conversation and have other stakeholders respond to it.”
I asked about voluntary guidelines for use of the Internet. The answer: PhRMA will wait for FDA guidance before it issues any further self-regulatory guidelines for the Internet as it did for print & TV DTC advertising. “Once the FDA acts,” said Francer, “we can then move to put those standards into effect and then we can take a look at PhRMA’s voluntary standards to make sure they are adequate.”
With regard to adverse event reporting, PhRMA cited “International Conference on Harmonisation (ICH) Tripartite Guideline E2D:”
- Sponsors “are not expected to screen external Websites for ADR information.
- However, if [a Sponsor] becomes aware of an adverse reaction on a website that it does not manage, the [Sponsor] should review the adverse reaction and determine whether it should be reported.
- [Sponsors] should regularly screen their Websites for potential ADR case reports.”
Later this week PhRMA will put forward the idea that FDA and FTC should “redouble their enforcement efforts against fraudulent activities on the Internet.”








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




