2015 is coming to an end, thankfully. It’s not been an especially good year considering that the media and politicians have found a face for evil pharma and rising drug prices: Martin Shkreli!
Shkreli’s recent arrest will only heighten pharma’s bad press as some people wonder why more pharma CEOs have not been arrested.
Be that as it may, let’s look ahead to 2016 and what I recommend as New Year’s Resolutions for the drug industry and especially pharmaceutical marketers. If you’re interested in how well my 2015 resolutions fared, go here.
My #1 personal New Year’s Resolution is the same every year:
Stop telling the pharmaceutical industry what to do all the time. Unfortunately, I have never kept that resolution. So, here’s my recommended list of 2016 resolutions for pharma…
#1: Put more real-world distance between you and the likes of Martin Shkreli
The drug industry is getting oodles of bad press related to the actions of Martin Shkreli.
“Shkreli has become the Wolf of Pharma Street — he’s basically come to represent everything that was bad and wrong with pharma,” said Art Caplan, a medical ethicist at New York University, as quoted in a Chicago Tribune article (here). And while Shkreli may be reviled, said Caplan, “he’s not doing anything in terms of prices that other companies haven’t done.”
Shkreli said he was doing nothing more than increasing shareholder value, a sentiment recently expressed by Pfizer CEO Ian Read, the REAL wolf of Pharma Street, who said pharma companies don’t make “undue” profits (see here).
In his first interview since he was charged for allegedly misleading investors in his hedge funds and raiding a public company to cover the losses, Martin Shkreli told the Wall Street Journal he had been targeted by authorities for his much-criticized drug-price hikes and over-the-top public persona. The U.S. Securities and Exchange Commission has been looking into Mr. Shkreli since 2012, though its probe became a higher priority this September after Mr. Shkreli raised the price of Daraprim, the Journal earlier reported.
Frankly, I’m getting tired of it all. Shkreli had his 15 minutes of fame. Let’s move on. It’s time for the pharma industry to get its 15 minutes of fame by doing something substantial to distance itself from the likes of Shrkreli.
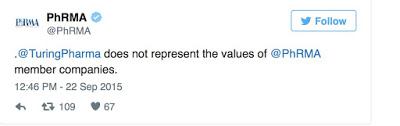
The mainstream drug industry cannot distance itself from “wolves” like Shkreli merely through better corporate communications (as suggested here) or with 140 characters or less on Twitter such as in this PhRMA tweet:
Actions are needed such as ending exploiting loopholes in the orphan drug act. Read, for example, “Otsuka [a PhRMA member company] to FDA: No Thanks… Orphan Status for Abilify is More Profitable. Generics Can Pound Sand!” [Would you believe that Crestor is an “orphan” drug? See here].
But unless pharma puts the brakes on ever-increasing drug price hikes (see here and here), Shkreli will be the industry’s poster boy.
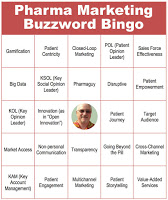
#2: Cut down on the use of buzzwords
Pharma marketers cannot communicate with each other without using “buzzwords.” But it may be a good idea to cut back a bit as some critics have suggested that buzzwords alienate patients and physicians (see, for example, “Do Marketing Buzzwords Affect Pharma’s Reputation Among Patients & Physicians?”).
According to results of a Pharma Marketing News reader survey (here), even industry insiders question the use of buzzwords. “We on the commercial side of the business have an unhealthy obsession with ‘buzzwords’,” said Timothy White, Senior Director & Head of Global Customer Interaction Management at Lundbeck.
One buzzword that pharma could do without is “Key Opinion Leader” (KOL). You can find the definition of that term here.
Nearly two-thirds (62%) of physicians deemed to be genuine medical experts believe the pharmaceutical industry should replace the term. That was one of the insights gleaned from an international online survey presented at the Medical Affairs Leaders Forum in Berlin, Germany, in February, 2015 (read “A KOL By Any Other Name Would Smell As Sour. What About POL?“).
#3: If you can’t do without direct-to-consumer (DTC) ads, at least get rid of those embarrassing “mascots!”
This is a follow-on to my 2015 Resolution #3: If you can’t break the TV DTC over-spending habit, at least stop broadcasting commercials that embarrass the industry as a whole. At that time I cited the new Viagra ads as an example of embarrassing DTC. The American public has now grown immune to those ads (read “Only 10% of Americans Would Pay Attention to Sexy Online Ads“) and the battle is lost as far as getting them off the air – even if a former Pfizer executive calls for it (for more on that, read “New Viagra TV Ad Should Be Dropped, Says Former Pfizer Executive“).
But perhaps more embarrassing are the TV DTC ads that feature characters like Xifaxan’s “Bubble Guy” who is supposed to look like your intestines but has been compared to a dinosaur and Jar Jar Banks from Star Wars. What could be more embarrassing than that? How about the first ever DTC ad — which may or may not have run on TV — that mentions the “V” word? For more on that, read “Pfizer’s ‘Humorous’ Menopause Ad Mentions the ‘V’ Word.“
See my Gallery of Drug Advertising Mascots.
#4: Stop inventing phony “real medical conditions” like Binge Eating Disorder (B.E.D.)
In February, 2015, Shire introduced one-time tennis celebrity Monica Seles, to help them hawk, er… market, a new indication for Vyvanse: Binge Eating Disorder or B.E.D. (see here).
According to Seles & Shire on the BingeEatingDisorder.com website:
“Binge Eating Disorder (B.E.D.) is not just overeating. It is a real medical condition [my emphasis] that was formally recognized in 2013. B.E.D. is the most common eating disorder among US adults.”
Although Shire’s website has lots of statistics about B.E.D., it does not provide the sources for these statistics. For example, Shire says B.E.D. affects 2.8 million people in the U.S. based on a national survey. But Shire does not say who did the survey or link to the source so we can see if it was based on real science or just marketing hocus pocus. I reported this back in February and still there is no link to the source of the claims made by Shire. Monica, however, is no longer featured on the site.
NOTE: According to the eHealth Code of Ethics, which I helped create, individuals need to be able to judge for themselves the quality of the health information they find on the Internet. Sites should disclose what sources the site or content provider has used, with references or links to those sources.
#5: Be (a lot) more transparent
EFPIA (the European Federation of Pharmaceutical Industries and Associations) has said: “The pharmaceutical industry recognises that it has a responsibility to show leadership in advancing responsible transparency.”
The importance of transparency was emphasized by several presenters at an industry conference I attended in 2015 (see here). But the drug industry has a long way to go to achieve “responsible” transparency and even to show leadership in advancing that goal.
For example, there has long been calls for the drug industry to be more transparent with regard to reporting clinical trial data. Lack of transparency about negative clinical trial data is a manifestation of the “dark side” of pharma R&D. Dr. Ben Goldacre, author of Bad Pharma, has written and lectured extensively about this (see “Bad, Devalued, Distrusted & Defensive Pharma: A Tale of Two Books“; also read “Results of Over 500 Clinical Trials Each Year Go Undisclosed to Public“). There is also calls for transparency regarding how drug companies set drug prices (read “Transparency Hits a Wall When It Comes to Drug Pricing“).
These are big, complicated transparency issues that are difficult to solve. However, it shouldn’t be difficult for the drug industry to be more transparent in its dealings with celebrities — including patient blogger celebrities. At the conference mentioned above I learned that transparency is good in theory, but not in practice when it comes to revealing payments to patient bloggers who “contribute” content to pharma-owned sites.
Given the power of celebrities and patient bloggers to influence people, pharmaceutical pharmaceutical companies should disclose the details of payments made to celebrities as they now are required to do for physician payments. Read more about that here: “Kim Kardashian’s Diclegis Instagram Post Raises Issues of Transparency“.
#6: Increase the quality (not quantity) of mHealth apps you develop for physicians and patients
There are plenty of hurdles to designing and developing mHealth apps that are truly effective at improving health outcomes and winning over physician prescribers.
Lack of evidence is just one of the hurdles to widespread provider prescribing of mHealth apps. Other hurdles include reimbursement challenges, limited integration with physician office systems, gaps in patient access, and, last but not least, data security and privacy issues.
“The fast-paced growth of the healthcare app market has outpaced the ability to develop oversight and guidance for accuracy of clinical content contained in mHealth apps,” concludes a report by IMS Institute for Healthcare Informatics (read “The Overwhelming Choices, Limited Proven Efficacy, & Security Issues of mHealth Apps“).
One way for the pharmaceutical industry to overcome these hurdles is to emulate Bayer Health, MSD, and Johnson and Johnson by collaborating with technology startups (read, for example, “MSD UK Joins #Pharma Digital Accelerator Club“). According to the recently published “Digital Doctor Report 2015” produced by Ipsos, “doctors have a greater level of distrust of pharma compared to technology companies.” Specifically, 40% of European physicians surveyed say they don’t trust applications developed by pharma. In contrast, only a quarter of physicians express the same sentiments toward technology firms (read “#Pharma Should Not Be Afraid to Develop Digital Apps to Improve Health Outcomes“).
So, not only can pharma produce better mHealth apps by teaming up with technology companies, they may also increase physician trust in the apps they develop, which will lead to more physicians recommending apps to patients to improve outcomes. Of course, those apps should be tested for efficacy just like drugs are tested for efficacy — via controlled clinical trials (see here).
#7: Invite Pharmaguy to give a presentation at your next “Digital Day” or other company meeting
It’s no surprise that social media teams from two of these companies received the Pharmaguy Pioneer Award.
That’s not to say you also will receive an award if you invite Pharmaguy to speak to your team. But you will get a chance to be interviewed by Pharmaguy and receive at least one complimentary, autographed copy of his book Socialize Your Patient Engagement Strategy, which makes the case for a fundamentally new approach to healthcare communication; one that mobilizes patients, healthcare professionals and uses new media to enable gathering, sharing and communication of information to achieve patient-centricity and provide better value for both organizations (in terms of profit) and patients (in terms of better service and improved health).
If you can’t invite Pharmaguy inside your tent, at lest you can get his book at a 35% discount off the usual price! Use code G15JNY35 when ordering here.















![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




