Mark Senak over at Eye On FDA Blog has analyzed 235 Warning Letters (WLs) and Notice of Violation (NOVs) letters issued by FDA’s Office of Pharmaceutical Drug Promotion (OPDP) since 2005. He cataloged 600 violations, including:
- risk omission or minimization,
- superiority claims,
- overstatement of efficacy,
- unsubstantiated claims and
- broadening of indication
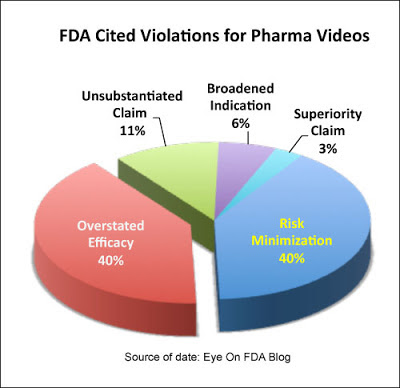
When Senak specifically looked at letters regarding pharma marketing videos (excluding TV DTC ad videos), he found that 80% of the violations concerned risk minimization (40%) or overstatement of efficacy (40%). Below is the remake of his pie chart of these data (for the original data see “Viewing Video’s Regulatory Profile“).
What’s interesting is that these videos — mostly patient and physician testimonials — overstate efficacy at TWICE the average for all kinds of promotions (40% for videos vs. 21% for all ads, including video). Senak postulates that “when people talk about their own experiences with a treatment, [they] may include reference to outcomes that is not typical or supported by clinical data.”
An example of a video that overstated efficacy was a video testimonial featuring Ty Pennington posted on youtube.com by Shire. FDA said “Both the webpage and video overstate the efficacy of Adderall XR; the video also omits important information regarding the risks associated with Adderall XR use.”
The problem with FDA letters is they usually are sent well after the cow has walked through the open barn door! See, for example, “Vyvanse Warning Letter: Too Late! Shire Got Rid of Ty Pennington Long Ago!“








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)