In preparation for FDA’s public hearing on the Promotion of Food and Drug Administration-Regulated Medical Products Using the Internet and Social Media Tools, the agency is asking for comments on 19 specific questions (see “Let’s Respond to FDA’s Questions Regarding Its Regulation of Social Media“). These questions are included in my ongong online survey/questionnaire, which you can access here.
I am following the results of this survey closely and will provide updates. Here, I focus on this question:
How should product information be presented using various social media tools to ensure that the user has access to a balanced presentation of both risks and benefits of medical products?
The survey asks respondents to choose ONLY one of the following responses (and add additional comments):
- No matter the media, all product ads should include major risk information along with benefits (media agnostic)
- When it is not possible to include major risk information due to space limitations, it is sufficient to include a link to the product Web site where consumers can then find all the necessary risk information in the package insert (2-click rule)
- When it is not possible to include major risk information due to space limitations, it is sufficient to include a link directly to the package insert (1-click rule)
- None of the above
For background on the “1-click rule,” see the following:
- “The ‘One-Click Rule’: Rant or No Rant?“
- Death of the One-Click ‘Rule’ or ‘Received Precedent’ or Whatever!
- Uh, Oh! Will FDA Cite this ‘One-Click Rule’ Twitter Post by AZ as Violative?
The image below shows how respondents answered this question (ALL respondents vs. PHARMA respondents vs. Agnecy respondents). The image will be updated frequently. See the date stamp on the image for when it was last updated. To see the most up-to-date results, please take the survey yourself and you will be able to see a summary at the end.
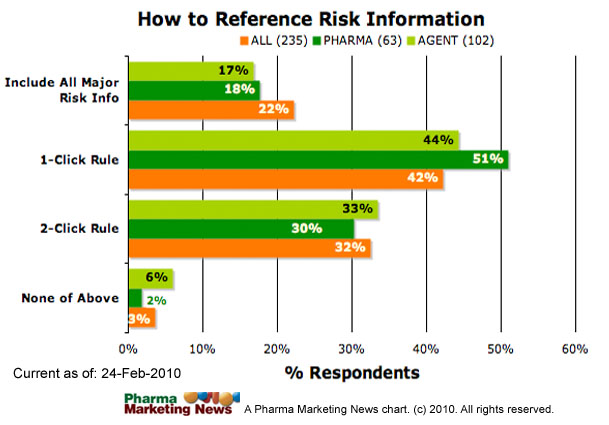
Several comments were submitted in response to this question. Some of these comments include:
- Ads should carry a disclosure of interest and warning about risks with a 1 click route to package insert
- Magazine advertisements, largely seen as the gold standard, require a page turn to review fair balance; a 1-click rule is a logical analog for the online world, and online ads must not be held to an unfair standard
- More than worry about providing risk/benefit info, FDA should worry about how to make this info more accessible/comprehensible to patients. Social media could be a great way to achieve this by providing patients a means to ask questions and by offering a much more comprehensive (visual, audio, video, graphs etc.) to share and communicate this important information.
- The majority of the public does not know that PIs exist let alone how to read them. Taking them to a company website allows for too much bouncing vs. immediate redirect to Patient PI
- Nevertheless it should be sufficient that FDA maintains an online database with all the package inserts accessible for any user at any time.
- The risks must be written in regular English and easily understood. Should have an initial summary as many people skim pages quickly.
- Paid Tweets seem out-of-bounds, to my experienced eye.
- in my experience, it takes a lot of space/characters to fully and fairly disclose all the risks/benefits, and it seems that if the user can get to it within two clicks that should be sufficient
- As may already be the case, abbreviated product inserts should be available (1-click rule) at “8th grade” reader level, focusing on the key risks and benefits of the drug or device. Also, updates of these information documents should be frequent and immediate if serious adverse events are verified.
- The one click should go to page which has package insert, but also synopsis of PI with major risk/benefit highlighted. PIs can be daunting for patients to navigate through.
- Another potential option is to link to a manufacturer-sponsored microsite that has been specifically designed to fit social media fair balance needs. In some situations, this could be customized to provide contextual fair balance to correspond to the topic being discussed in the social media vehicle. (eg. a discussion board comparing side effects between products links to a microsite that provides all approved messaging regarding comparative data, along with a disclaimer that any other claims or data has not been reviewed or approved by the FDA)
- I’m a 2-click rule advocate with easy-to-find access to PI.
- A 1 click rule where the user lands on a ‘landing page’ that includes the PERTINENT fair balance info would be good too. This would be user-friendly (since it’s not the entire PI) and also very informative (since it pertains to the parent content).










![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




