According to the most recent Harris Interactive poll the “Reputation of Pharmaceutical Companies, While Still Poor, Improves Sharply for Second Year in a Row.”
“Every year, Harris Interactive measures the public’s perceptions of approximately 20 industries. Specifically, we ask people to say if they think each industry is generally doing a good or a bad job of serving their consumers. It is clear that answers to this question reflect not just the specific wording, but many general public feelings about the different industries. If people feel good about an industry, they tend to say it is doing a good job of serving its consumers and vice versa.”
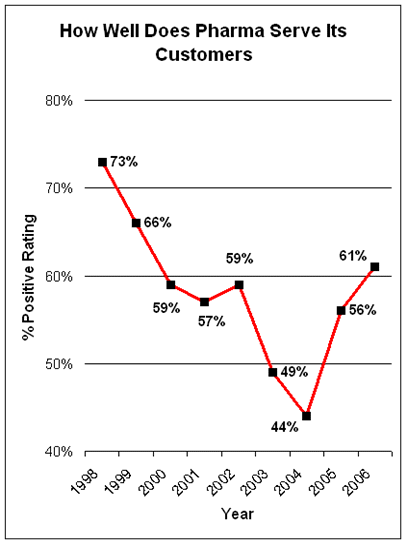
Here’s a chart of the poll results since 1998:
The recovery is no fluke, but what could possibly be the cause of this reversal of fortune? According to Harris Interactive:
“First of all, it is important to recognize that the pharmaceutical, managed care and health insurance industries are stilled rated very near the bottom of the list in terms of public approval. They are not popular, but they have become less unpopular. A plausible explanation of their upward trend is that they have been receiving less unfavorable media coverage and publicity over the last two years than previously.”
“In the case of the pharmaceutical industry, the massive decline from plus 60 percent in 1997 to minus four percent in 2004 was surely a direct result of the constant drumbeat of unfavorable publicity about their prices. While drug prices are still a major issue for many people, there has probably been less negative publicity on this issue over the last two years than previously.”
Medicare Part D
I believe that the new Medicare Part D drug benefit had a big role in both the “dip” in the ratings data in 2003/2004 and the recovery in 2004/2005.In 2003 and 2004, the drug price debate was in full swing primarily because the Medicare bill was wending its way through Congress. Everyone was talking about the high cost of drugs.
That was then. Now that Part D is “fully operational,” there is less direct talk about drug prices — most of the current criticism of the drug industry is at least one layer away from the pocket book (DTC, gifts to physicians, etc.). Moreover, despite the criticisms leveled against the program, now that it is law, Medicare Part D represents a drug price safety net for many people. The program also is endorsed by AARP, which was once an outspoken critic of prescription drug prices, and now touts the program as saving money. Medicare Part D also serves to deflect attention away from the drug industry and onto the government and insurers as far as drug prices are concerned.
Drug Safety
Harris Interactive says “Another interesting point is that the bad publicity in relation to drug safety problems does not seem to have had much impact.” I don’t know how they can say that. There is no doubt in my mind that the Vioxx withdrawal debacle had something to do with the continuation of the rating dip in 2004. In 2005 and 2006 there were no major drugs pulled from the market due to safety concerns and therefore drug safety was not top of mind as far as the general public was concerned. The FDA even pulled the plug on its plan to implement a Drug Watch Web site that would have listed drugs with safety issues. No one raised any hue and cry over that. [For more on the FDA’s proposal, see “FDA Drug Watch Site Guidelines.”]Other factors to consider with regard to the rise in the favorable rating in 2005 and 2006 include:
- Increased Lobbying (see “Activists: Drug firm lobbyists ‘blanket’ Congress“, Boston Globe)
- Grassroots campaign by industry representatives (see “GSK Strikes Back with a Grassroots Campaign” and “GSK’s ‘Plain Talk’ Flawed“)
- Merck’s “Patients First” TV campaign (see “Patients Come First?“)
- DTC Guidelines issued by PhRMA in 2005 (see “PhRMA Finalizes DTC Principles” and “Less DTC, Better Public Image?“).
Lobbying is a Factor
According to a Center for Public Integrity investigation (“Drug Lobby Second to None: How the pharmaceutical industry gets its way in Washington“), 2003 and 2004 were banner years for pharmaceutical lobbyists:“In 2003 alone, the industry spent nearly $116 million lobbying the government. That was the year that Congress passed, and President George W. Bush signed, the Medicare Modernization Act of 2003, which created a taxpayer-funded prescription drug benefit for senior citizens.
“That figure was not anomalous. In 2004, drug makers upped their reported expenditures on lobbyists to $123 million, a record amount for the industry. Of the 1,291 lobbyists who were listed that year as presenting pharmaceutical corporations and their trade groups, some 52 percent were former federal officials.”
All this lobbying paid off in 2005 and especially in 2006 when Medicare Part D became effective.
Another target of pharmaceutical lobbyists that has paid off with good public relations concerns bioterrorism. The pharmaceutical industry has been called upon to protect the American public from biological attack by producing vaccines and other pandemic protective drugs. Drug industry lobbying, according to Public Citizen, was effective in getting the federal government to shield it from lawsuits over products used to treat pandemic illnesses (see “Willful Misconduct: How Bill Frist and the Drug Lobby Covertly Bagged a Liability Shield“).
I am glad that the pharma industry is there to provide this protection, although I am not sure about the shield. Obviously, the industry has scored good PR points for the former while the latter has been swept under the PR rug.
Industry Counterattack
Meanwhile, the drug industry is counterattacking to directly influence public opinion. Mike Pucci, Vice President of External Affairs at GlaxoSmithKline (GSK), for example, heads a program whose goal is to get the word out about the good that the pharmaceutical industry is doing (or, as Pucci expressed his goal: to “Restore the reputation of the industry by communicating the value of our products, our research and our hope for the future”). In presentations on his tactics, Pucci claims a lot of the credit for the improved public perception evidenced in the Harris Interactive survey poll numbers (see “GSK Strikes Back with a Grassroots Campaign“).Merck also is fighting back. The company is spending at least $20 million on its “patients come first” campaign which I criticized in this blog some time ago. “The ads seek to counter perceptions among skeptical consumers that Merck in particular — and giant drug makers in general — are more interested in profits than in people” (see “Merck to spend $20 million to buy your trust“).
I’ve read that Merck considers this campaign effective in improving its public image. If the campaign has helped Merck, then it no doubt has helped other drug companies as well. After all, Merck is the poster boy for the bad, the ugly, and now (apparently) the good side of the industry.
PhRMA and DTC Principles
Part of the counterattack by the industry has been PhRMA’s Guiding Principles on DTC Advertising. While these principles have no enforcement teeth (see “Wake Up PhRMA! or Tilting at Windmills“), they have been a boon to the industry’s public image and quelled a lot of the debate about DTC — especially in Congress. As time goes on, however, I expect less and less attention will be paid to the “spirit” of these principles (e.g., less unbranded disease awareness ads, for example). In fact, the battle is on to make DTC even more effective by simplifying the risk information they need to communicate (see, for example, “DTC without the Risk“).Aside from the DTC guidelines, PhRMA is playing a larger role in cleaning up the industry’s public image since Billy Tauzin became president of the organization in January, 2005. Since then, for example, the PhRMA Web site has had a major overhaul and is much more consumer friendly.
Is this as good as it gets? Or will the positive ratings continue to rise? It’s hard to tell, but I suspect that a 60% positive rating is just about right for the industry these days. After all, how many Republicans are there?









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




