In preparation for FDA’s public hearing on the Promotion of Food and Drug Administration-Regulated Medical Products Using the Internet and Social Media Tools, the agency is asking for comments on 19 specific questions (see “Let’s Respond to FDA’s Questions Regarding Its Regulation of Social Media“). These questions are included in my ongong online survey/questionnaire, which you can access here.
I am following the results of this survey closely and will provide updates. Here, I focus on this question:
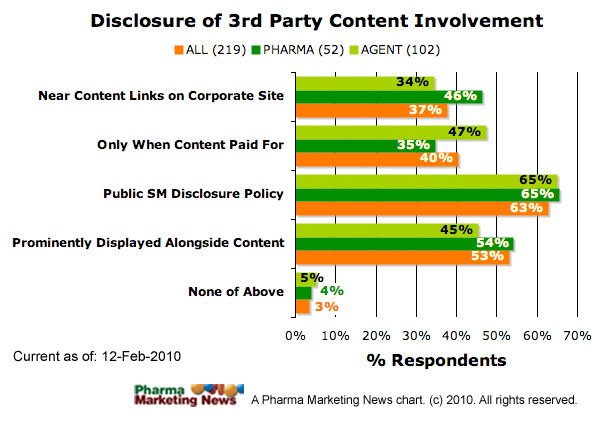
How should companies disclose their involvement or influence over discussions or material, particularly discussions or material on third-party sites?
The survey asks respondents to choose one or more of the following responses (and add additional comments):
- Disclosure is necessary only when content is paid for.
- Disclosure should be prominently displayed alongside relevant content when possible
- Disclosure and disclaimers should be included prominently on the corporate website near any links to social media outlets.
- Each company should have a public SM policy that includes a notice of its transparency policies.
- None of the above
The image below shows how respondents answered this question (ALL respondents vs. PHARMA respondents). The image will be updated frequently. See the date stamp on the image for when it was last updated. To see the most up-to-date results, please take the survey yourself and you will be able to see a summary at the end.
Many comments were submitted in response to this question. Some of these comments include:
- We can’t make rules/laws for everything. It would be better to hold companies responsible to their stakeholders – investors, customers, patients, healthcare professionals, etc etc.
- Disclosure and disclaimers should be included prominently on the corporate website near any links to social media outlets.
- When companies pay for content to be produced on their behalf, a disclosure, disclaimer and list of policies should be included prominently on the corporate and associated websites near any links to social media outlets.
- A notice of disclaimer should be provided whenever there is a content favorable to the company’s interests that has been elaborated directly or indirectly by any third-party benefited by the company.
- Disclosure must be constant, no exceptions; even Twitter comments must contain disclosure, even if in the form of a hashtag (#iwork@novartis is in use today)
- If true: “We encourage our employees to message about their experiences with our products, but they are not separately compensated for these messages. You should consider whether opinions being offered here are from a source whose employment is tied to the maker of the product being discussed.”
- If company involved, should always disclose. In scenario where a brand has sponsored some activity, could disclose pharma company involvement without mentioning the brand name, not as a way to cover up brand involvement but to avoid using brand name and triggering need for fair balance.

SPECIAL REPORT: FDA Regulation of Social Media
Find links to more preliminary results here.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




