California leads the way in many things, including laws to reign in pharmaceutical marketing and sales. Where California leads, many other states follow. The latest proposed California law is Assembly Bill 2856, introduced February 24 by State Assemblywoman Loni Hancock (D.-Berkeley). More on that below.
Gifts to Physicians
Another recent law enacted in California in 2005 (the California Health & Safety Code 119400-119402) requires pharmaceutical companies to declare a limit on the amount of money they spend on “gifts” per physician in California. This subject is discussed by experts and survey respondents in the latest issue of Pharma Marketing News (see “Free Gifts to Physicians: What’s the Big Deal” in the February 2006 Issue).
According to the February 17, 2006, Daily Health Policy Report published by Kaisernetwork.org, “at least nine states are considering bills that would require pharmaceutical companies to publicly report annual gifts to physicians, hospitals and pharmacists…”
[Actually, Vermont, was ahead of California with a 2002 law that requires drugmakers to report to the state’s attorney general all gifts of $25 or more given to doctors, hospitals or pharmacists. (see “What Pharma Companies Spend on Gifts to Docs“). It may just prove my point: States like Vermont are vying to beat California, which is still seen as the leader in the fight against big pharma.]
Transparency
Thanks to these laws, the industry is forced to be a bit more transparent about where its marketing dollars are spent (see table below). Massachusetts state Sen. Mark Montigny (D), sponsor of a Massachusetts bill patterned after the Vermont law , said, “If a doctor needs a Caribbean vacation or a mug or a pen, he or she is probably not very successful and needs to be in another business.” Montigny added, “The No. 1 thing that keeps government and corporate officials honest is transparency.”
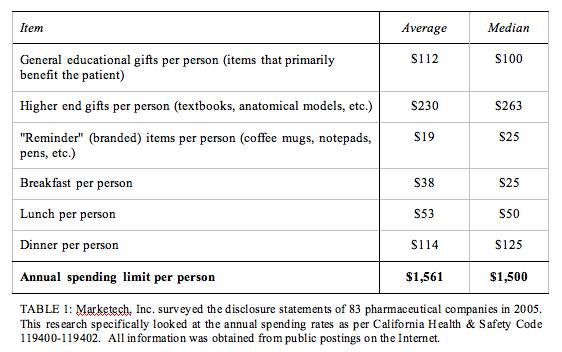
I agree with the need for transparency to keep people honest (see “New Year’s Resolutions for Pharma“), but I prefer that companies do this on their own rather than it being legislated. Unfortunately, it takes a crisis to get pharma companies to adopt voluntary guidelines. By then it is often too late to stop the wheels of government regulation.
AB 2856: Off-label Practices Attacked
AB 2856, aka, Prescription Medication Off-Label Use Informed Consent, would require physicians and surgeons (not dentists?) to obtain informed consent from patients before prescribing, administering, or furnishing prescription medications for off-label uses. It defines off-label as: “prescribing, administering, or furnishing a prescription medication to treat a condition that is not within the indications for that medication approved by the federal Food and Drug Administration.”
“Before prescribing, administering, or furnishing a prescription medication for an off-label use,” says AB 2856, “a physician and surgeon shall obtain informed consent from the patient. For these purposes, the physician and surgeon shall inform the patient verbally, in easily understood terms, of all of the following information:
- The medication is furnished to treat a condition that is not within the indications approved for that medication by the federal Food and Drug Administration.
- The nature, degree, duration, and probability of the side effects and the significant risks of the medication that are commonly known by the medical profession, including the medication’s adjuvants, and the degree to which the side effects of the medication may be controlled.
- A division of opinion exists as to the efficacy of the use of the medication.
- A description of the effect of the medication on the human body.
- The dosage deemed medically necessary to treat the patient’s condition.
- The median age group for which the medication is prescribed.
- Available and appropriate medical alternatives to the medication and the reasons the physician and surgeon recommends the medication instead.”
This is quite a laundry list! In this day and age when pharma companies are emphasizing that all drugs have risks and that it is up the informed patient to evaluate drug risks vs, benefits, the above procedure should be applied to ALL drugs, in- or 0ff-label.
“Armed with the knowledge that a prescription was off label, proponents say, patients might ask more questions; seek other sources of information, such as the Web; watch more closely for side effects; or ask for an approved treatment instead.”
But will physicians comply if the law was passed?
“But doctors say such a disclosure would only scare patients, possibly spurring them to refuse a medicine they sorely need.” San Francisco Chronicle: Hard Sell: How Marketing Drives the Pharmaceutical Industry” (May 2005).
You can expect both physicians and the pharmaceutical industry to weigh in on the merits of this bill now that they can see the language.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




