In a story about the struggle of Amarin Pharmaceuticals against a “Fish Oil Backlash,” Forbes blogger Matthew Herper said “the idea that some drugs may improve laboratory values without helping patients is now popular among cardiologists” (read here).
Herper suggested that this “popular” belief has hurt prescriptions for niacin, sold by Abbott, for Merck’s blockbuster cholesterol pills Zetia and Vytorin, and now for Lovaza, marketed and sold by GSK.
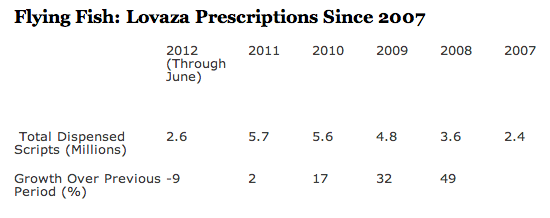
At first, GSK’s marketing “prowess” seemed to overcome common sense; i.e., that you might as well get your fish oil from less expensive OTC supplements or from fish itself (see “Proof That Pharma Marketing Works: Lovaza sold more than $1 B in 2010!“). But as the following Lovaza Rx data shows, the blockbuster days may be over for Lovaza:
It turns out, however, that marketing “prowess” may have had less to do with the rise of Lovaza than ancillary “unsavory and aggressive” tactics such as “hiring doctors with little expertise in heart disease to talk up Lovaza’s cardiovascular benefits, paying one primary care doctor $45,000 over a three-month period to speak about the medicine,” according to an expose cited by Herper.
Is this another incidence of GSK inappropriately influencing prescribing decisions?
According to a Pharmalot blog post (here) that exposes other GSK unsavory marketing practices associated with Advair (here), a Glaxo spokeswoman said that “it is absolutely against GSK’s policies and practices to inappropriately influence prescribing decisions.” The spokeswoman added that such practices do not reflect “the company we are today.” That, IMHO, remains to be see.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




