Medical Device DTC stats was the topic of an interesting discussion thread last week on the Pharma Marketing Forum — a good place to get answers on all kinds of issues relating to the pharmaceutical industry.
Medical Device DTC stats was the topic of an interesting discussion thread last week on the Pharma Marketing Forum — a good place to get answers on all kinds of issues relating to the pharmaceutical industry.
As often happens, the discussion was opened up by a simple question:
“Is anyone aware of any research that has been done around what sources were used by and how much a medical device companies spend for advertising / promotions / DTC / brand marketing, etc.? This information is easy to find for the pharma side, but it’s more difficult on the device side.” — Melody
My friend, Bill Trombetta, is always a knowledgeable source of information and had this suggestion as a direct answer to the question:
“I am starting to do work with med device companies. They do very little DTC and “traditional” marketing. For ex., Stryker, orthopedic devices, is using Jack Nicklaus in DTC, a first for a very conservative industry sector.
“I don’t know of any source that beaks out the promo mix as detailed as you have set it out. But you might try pulling up a company’s 10 – K and going to its Income statement and there might be a category for marketing spend overall.” — bill trombetta, professor of pharmaceutical marketing, st joseph’s university
David Jastrow, Senior Research Analyst at Thomson Scientific & Healthcare, donated some specific information:
“The 10-Ks have bits and pieces of info that may be useful. Medical device companies are still sorting out best practices for DTC, it will be interesting to see how they apply lessons learned from the pharmas to hopefully improve upon the model. The following rough slides may be of some interest to you.” — David
The following is excerpted from David’s slides with my notes interspersed:
Medical Device Companies Face Greater Scrutiny
Recognizing the lack of attention that has traditionally been paid to regulating direct-to-consumer (DTC) marketing of medical devices, FDA in November 2005 held a public hearing to seek input on whether the agency needs to revise its approach to the promotion of medical products, including restricted devices.
For more on this meeting see:
- DTC Pros and Cons Presented at FDA Hearing
- FDA DTC Hearings: Snippets from Day 1
- FDA DTC Hearings Day 2
[For all blog topics relating to the FDA and regulation of pharmaceutical marketing, see Food and Drug Administration (FDA).]
Testimony at that meeting included the following statement:
“The device industry is relatively new to the game,” says Carolyn Jones, associate vice president of technology and regulatory affairs with AdvaMed [trade association representing medical device manufacturers]. “Drug companies have had a lot more experience with DTC advertising. Our suggestion to FDA is that devices need to be viewed in a little different light. The medical device industry has a good track record, although limited, with respect to DTC advertising. Because the medical device industry is a late-comer to the game, it’s been able to learn from the mistakes of others. If it isn’t broke, hopefully they won’t try to fix it.”
Factors Influencing Med Device Product Development
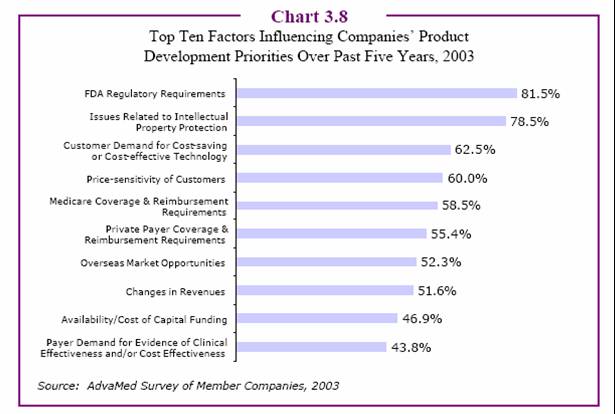
[According to the above survey data, medical device companies may be more concerned with price issues. Obviously, devices are much more expensive than drugs and it is amazing to me that medical device companies are actually beginning to run DTC ads for hip and knee replacements. I don’t recall much fair balance in these ads — what, if any, problems may be associated with these devices? — JM]
Device Manufacturers Spend Less on R&D, Gain More Patents
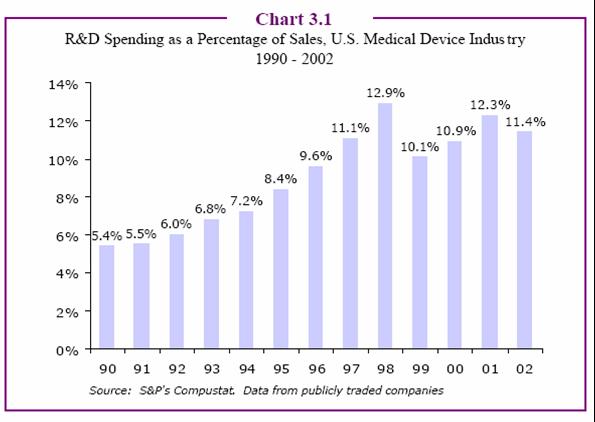
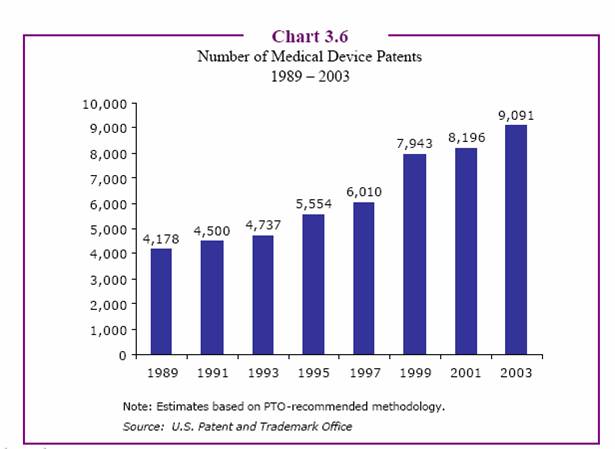
[R&D spending by device manufacturers is probably a little less than that of drug companies as a percent of sales. However, as the next chart shows, the number of device patents continues to increase year over year. The drug industry, on the other hand, shows a reverse trend. — JM]
Understanding the Patient Point of View
Continuing with the discussion thread:
“Many device companies don’t seem to feel a need to understand the patient POV, at least not yet. I met with some MR folks recently at one of the largest device companies, and some of them were very categorical about not feeling the need to investigate patient perspectives when conducting developmental research. ‘it isn’t done that way here’ was the attitude. But one of them pulled me aside and said ‘it’ll probably take a few years, but we’ll eventually get to a model where the patient’s perspective counts for us… It’s going to be a long process though.’ It’s not about changing the product to meet the ‘consumer’s’ needs, but it’s about anticipating positive and negative responses, in particular, that initial conversation when the possibility of a device is brought up in the office or at bedside. That isn’t done yet on a broad scale it seems.” — John Mitchel
“Our company has spent many years trying to gather the patient’s point of view with our device which shows in its design and treatment schedule. The constant need for our medical device and traditional drug therapy is compliance. We ask for only 20 minutes/day for five days a week and they still balk. People want instant results and lack patience in medical treatments.” — Leo
Compliance, compliance, compliance. How many times have I heard that tiresome phrase spoken by pharmaceutical marketers, who typically blame the laziness of patients? The word “compliance” itself reveals this top-down mentality. To truly understand the patient’s POV, marketers must admit that perhaps some of the fault lies with the product rather than with the patient.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)