Take a look at the Juvederm XC ad, which I found in this week’s New Yorker Magazine, which (coincidentally) includes an article about treatment of wrinkles (“Face It: The Truth About Wrinkles”). The article does not mention Juvederm and hardly has anything at all to say about injection of gels into the skin.
Do you think this ad is “fair and balanced”; ie, presents risk information that is “comparable in depth and detail with the claims for effectiveness or safety” as required by the Federal Food, Drug, and Cosmetic Act?
FDA issues “notice of violation (NOV)” letters to drug companies when it feels that promotional pieces overly minimizes risk information.
Should FDA send a NOV letter to Allergan, which markets Juvederm?
“Promotional materials are misleading,” said FDA in a recent letter to Meda Pharmaceuticals regarding an Astelin promo piece (see “FDA Warns Meda Pharmaceuticals that Astelin Isn’t Approved as a Cure for House Cleaning!“), “if they fail to present information about risks associated with a drug with a prominence and readability reasonably comparable with the presentation of information relating to the effectiveness of the drug.”
With regard to the Astelin promo piece, FDA noted “the only risk information contained on the sign (a disclosure of common adverse events) is presented at the bottom of the sign after the indication for the drug in extremely small font size and in a single-spaced format that makes this information very difficult to read.”
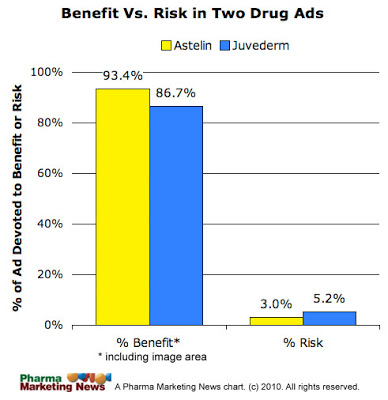
Clearly, the Juvederm ad also presents risk information in hard to read “mouse typeface.” But let’s take a more scientific, quantitative approach. I compared the area devoted to risk information versus benefit information in both the Astelin and Juvederm ads. My thesis is that there is a similar quantitative de-emphasis of risk information in the latter as in the former and that Allergan should also receive a letter from the FDA.
I’ve collected benefit vs. risk information data for a number of print DTC ads and have found that, on average, Rx drug print ads devote 65.3% to benefit information (includes image area) and 11.8% to risk information (not including information on back of the ad). See “Print DTC: How Does It Measure Up?: A Quantitative Analysis of Risk vs. Benefit Information.” Clearly BOTH the Astelin and Juvederm ads devoted MUCH less of the ad space to risk information than the AVERAGE print DTC ad.
I don’t know if the FDA will send a letter to Allergan or not. FDA has sent letters regarding violations that seem as trivial as this one may be. I’ll be keeping an eye out.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




