A new Competitive Enterprise Institute (CEI) analysis cautions that FDA’s current policy restricting use of Internet sponsored links could have the unintended effect of making regulated information less available to consumers and making less reliable and unsubstantiated information more available.
CEI’s report, entitled “DDMAC’s Internet Stance Could Increase Consumer Risk,” contends that “FDA’s position on sponsored links could have the unintended effect of making regulated information less available to consumers while making less reliable and unsubstantiated information more available…If sponsored links for highly regulated drugs and devices become more rare,” says CEI, “then an information seeker will be relegated to wading through a list of Web sites that contain all manner of information of dubious validity.”
Indeed, sponsored links for regulated drugs HAS become more rare (see “FDA Letters Caused a Prompt, Precipitous, & Prolonged Drop in Pharma Paid Search Engine Advertising“).
But CEI’s claim that the void has been filled by ads of “dubious validity” is unsubstantiated by any PROOF whatsoever. In fact, it is an example of “Carefully Wrought Bullshit” (see “Is Pharmaceutical Marketing BS?“) that displays a complete disregard for facts.
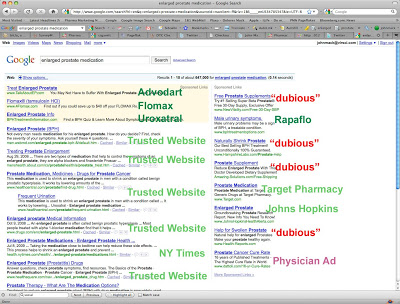
I did a little experiment to test CEI’s hypothesis. I searched Google for “enlarged prostate medication.” Below is the screen shot of the results. I checked out each of the results — both the paid links and the natural search results and labeled them in the following image (click for an enlarged view).
Four of 13 paid ads (31%) were for FDA regulated products. In fact, 100% of the paid ads at the top of the page are for such products. There are 4 ads of a “dubious” nature and 1 for a physician hawking his services (not sure if that is dubious or not. CEI says that physicians are “learned intermediaries” that protect consumers from any harm DTC advertising might cause.)
All the natural search results refer to trusted health websites that carry seals of approval or for a major newspaper (NY Times).
I have done similar searches before and have gotten the same results. The vast majority of paid and unpaid search results (72% in this case) will lead consumers to trusted and/or FDA-regulated health information.
CEI should not try and scare people by implying that FDA’s policies are harming public health!
P.S. Some of my colleagues are offering proof that when major Rx brands stopped advertising on Google, competitors — including those of a dubious nature — took up the slack as far as paid links are concerned. They use tools such as SpyFu, which is an “online competitive intelligence application that allows you to browse competitors’ PPC [pay per click; eg, Google Adwords] keywords, daily budget, bid prices, clicks/day, and other interesting PPC facts & figures” (see here). Using this tool you notice that when Rx ads are AWOL, you get competitor ads. That’s to be expected — this is a capitalistic, competitive society folks! When it comes to commercialism — dubious or not — it is always BUYER BEWARE online and OFF! Also, don’t forget that you still have natural, unpaid search results, which 75% of the time will lead consumers to reputable sources of health information online.
Another point about CEI’s report is that it sounds more like an anti-FDA screed than a rationale argument, which is something that won’t sit well with FDA folks. Accusing the FDA of harming the public health without any basis in fact is a slap in the face to many public servants who make considerably less income than the authors of the CEI report. Many within the FDA believe fervently in their mission to protect the public health and I can’t imagine them being very happy with CEI’s accusation.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




