That’s my re-interpretation of Josh Bernoff’s presentation “How to do a social application in life sciences without getting fired” at yesterday’s Social Pharmer Boston Unconference organized by @Shwen.
Bernoff is the co-author of the book “groundswell – winning in a world transformed by social technologies” and is Vice President and Principal Analyst at Forrester Research.
Bernoff’s title speaks to corporate politics. My re-interpretation tries to bring in the target audience of marketers — especially consumers and patients. If not approached correctly, SM denizens can really “blow up” any marketer’s carefully laid plans to engage them in conversation.
Stuart Foster, an attendee of the SocPharm unconference, summed up Bernoff’s presentation in his blog post:
“It came down to this: Your customers are going to talk and you can provide the space or you don’t. But conversation will continue with or without you. It’s just harder to facilitate once ball gets rolling in order to launch self serving community.
“Josh then laid out the basic model of: Consumers want to help. Pharmaceuticals want to help. FDA in the middle.”
Benoff’s presentation, which you can find here, includes the following graphic to illustrate his concept of FDA blocking pharma companies from connecting with consumers and physicians via social media:
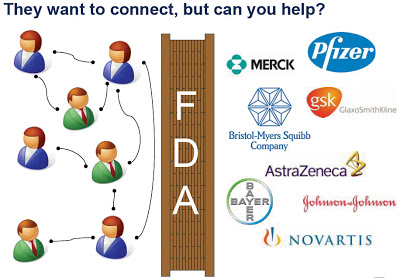
This image resonates with my previous post, “FDA, Tear Down This Wall! A Draft Petition Calling for a Public Hearing.”
Neither Bernoff nor I expect the FDA to get completely out of the way. I think we are in agreement that the FDA must show pharma a clear path around the wall, which should only inhibit the “bad guys” from getting through. Right now, however, some “bad guys” have a clear path to social media to promote all sorts of elixirs and worthless information about dangerous products.
How can FDA help pharmaceutical companies provide useful, balanced product information via social networks? Guidelines, guidelines, guidelines! Guidelines are the path around the wall.But there are many possible paths around the wall — not just any path will do. It must be the correct path and especially a path that EMPOWERED PATIENTS — part of the groundswell that INVENTED social media — decide is appropriate or at least acceptable. Consumers want to help, but not if they are kept out of the loop when it comes to developing the ground rules of engagement.That is why I believe a PUBLIC HEARING that will include patient advocates and other stakeholders is important BEFORE FDA and the drug industry decide among themselves what the path will be.
If guidelines are developed with input from all stakeholders, the drug industry will begin its journey around the wall and find the citizens there greeting them with flowers, not roadside bombs!
SURVEY: Should FDA Convene a Public Hearing on Use of Social Media by Pharma?








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)