There’s been a lot of brouhaha over the perception that the FDA is currently “risk adverse” and too quick to require “black box” warnings on Rx drug labeling (see these articles in Pharma Marketing Network’s “Industry News” Forum). The FDA uses the black box as a blunt instrument to ensure that drug marketers adequate communicate major risks to physicians and consumers. Physicians may read the drug labeling — though I’m not sure about this because I cannot recall any studies that show that physicians DO read the labeling — but it is pretty well established that consumers never see the labeling (aka package insert or PI) let alone read it.
The issue of communicating drug risk to consumers was covered in the April, 2008, issue of Pharma Marketing News (see “DTC Risk Communication“). This article summarizes the discussion I moderated during a panel session at a recent conference. The experts on the panel included: Scott M. Lassman, Partner, FDA Group, WilmerHale LLP; Hugo Stephenson, President, iGUARD.org; and Harry Sweeney, formerly Chairman, Dorland-Sweeney. I’ve interviewed Dr. Stephenson about iGuard in a Pharma Marketing Talk Podcast (listen to it here: “An Innovative System for Communicating Drug Risks to Patients“) and Harry is a long-time friend and member of my Roundtable of advisors (see here). I met Scott Lassman for the first time at this conference. Prior to his position at WilmerHale LLP, Lassman served as Senior Assistant General Counsel for the Pharmaceutical Research and Manufacturers of America (PhRMA).
Side Bar: Scott recalled my name because while working at PhRMA he heard of my PhRMA Intern blog posts and he told me that the posts drove “Emily,” the hapless REAL PhRMA staffer who famously responded to my inquiries with an amateurishly-written, extremely late reply (see “PhRMA’s Response – PRwise, it Stinks!“), to eventually leave PhRMA!
Harry’s Drug Risk-o-Meter Parlor Game
Harry Sweeney suggested that before you can communicate risk and determine who should communicate risk to whom (FDA to drug company, FDA to consumers, drug company to consumers, drug company to physicians-then to patients, FDA to physicians-then to patients, etc.), it is necessary to define what we mean by risk.
“We ought to clarify that risk is a lot more than odds and probability,” said Sweeney. “You have to have a hazard. You have to be exposed to the hazard. Then there’s proportion — the magnitude of the risk, which is not discussed in the pharmaceutical world. We are given tiny numbers upon which to make important major communications policy decisions.”
Sweeney pointed out the need for a baseline of what consumers understand about drug risk before we can move the needle where it ought to be. To illustrate what he meant, he engaged the audience in a “parlor game:”
Imagine a scale in front of you with a zero at one end and a ten at the other. Zero is extremely risky (not safe) and ten is extremely safe. Put your finger somewhere on the scale in answer to the question: “How safe are prescription drugs?”
“Imagine doing this in a roomful of people more representative of the general public,” said Sweeney.
Well, since the article was published, I have been inviting people visiting the article summary page to participate in Harry’ Drug Risk Parlor Game (a one-question online survey) and here is a plot of the results so far:
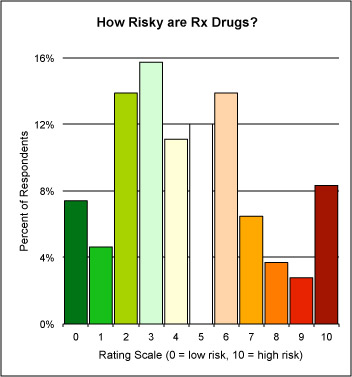
There are not enough responses to make this even close to scientific, but I note a peak on the “risky” side. I’d like to have many more responses to get a better sense of how risky readers thank drugs in general are, so you are invited to play Harry’s Drug Risk Parlor Game online here.
Thank you!









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




