In preparation for FDA’s public hearing on the Promotion of Food and Drug Administration-Regulated Medical Products Using the Internet and Social Media Tools, the agency is asking for comments on 19 specific questions (see “Let’s Respond to FDA’s Questions Regarding Its Regulation of Social Media“). These questions are included in my ongong online survey/questionnaire, which you can access here.
I am following the results of this survey closely and will provide updates. Here, I focus on this question:
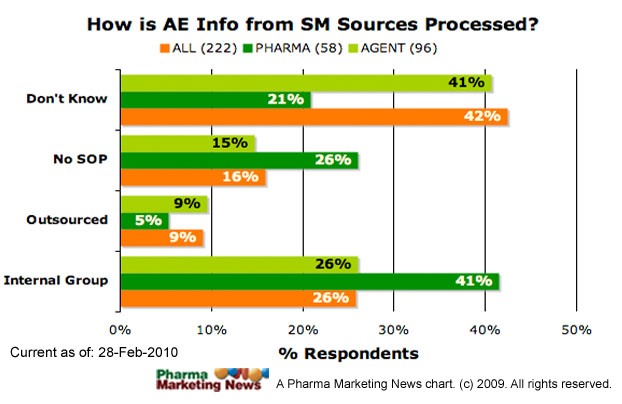
How is adverse event information from these sources being received, reviewed, and processed?
The survey asks respondents to choose ONLY one of the following responses (and/or add additional comments):
- Special group within the company is responsible for receiving, reviewing, and processing AEs (Internal Group)
- Receiving and processing AEs is outsourced to a specialized agency; review is handled in-house to determine which AEs need to be reported as required by law (Outsourced)
- The information is usually incomplete and does not meet the requirements for submitting a meaningful AER (not actionable)
- We have no SOP (standard operating procedure) for receiving, reviewing, and processing AEs from these sources (No SOP).
- None of the above
- Don’t Know
Of course, this question is most appropriate to be answered by someone within a pharmaceutical company. Some outside vendors/consultants may also know from their own experience working for pharmaceutical companies.
The image below shows how respondents answered this question (ALL respondents vs. PHARMA respondents vs. Agency respondents).

SPECIAL REPORT: FDA Regulation of Social Media
Find links to more results here.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




