Kim Kardashian posted a violative Diclegis® ad on Instagram (read “Kim Kardashian’s Diclegis Instagram Post Raises Issues of Transparency, Drug Safety, and Learning from History”). She was paid to do that by Duchesnay — the company that makes & markets Diclegis.
But did you know that the ad also was posted to her Twitter and Facebook accounts?
Here’s how the FDA depicted the campaign (find the original here):
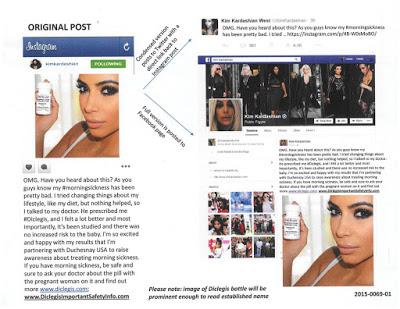 |
| Click on image for an enlarged view. |
What does this show us? How did the FDA get so social media savvy? Note two things:
- The Twitter post was only 3 hours old when it was captured in the above image.
- The Facebook post also must have been posted just before the image was captured because it is at the top of Kim’s page. It appears that the FB post was only 2 hours old when captured.
All these posts were made on July 20, 2015 and I found out about the Instagram post the next day (see “OMG. Kim Kardashian Shills for Pharma! No Worry – No Side Effects!“). It seems, however, that someone knew about these posts hours after they were made. Was that “someone” an FDA employee, a physician, or a competitor?
FDA says it was notified about the Instagram post through its Bad Ad program. Only physicians can submit complaints to that program (read “Is There a Doctor in the House? FDA Bad Ad Program is Designed for You. Not So Much for Me!“). What physician would be so quick in noticing the post, capturing the evidence, and submitting it to the FDA? Could it be a physician working at a competing drug company?
I know that FDA requires all drug companies to submit any promotional material to FDA’s OPDP “at the time of first use” together with a form called Form 2253. Companies have the option to do this before the ad is run and perhaps that alerted the FDA to keep an eye out for Kim’s posts — see P.S.
It’s interesting that FDA was able to act so quickly in the social media realm because with TV ads, it takes many months before the FDA reacts (read, for example, “FDA and YAZ: Is FDA Helping Marketers Work Around Regulations?“).
P.S. John Driscoll (@OPDPresources) just pointed out to me that the image is a copy of what was submitted to OPDP by the company with form 2253. The letter states: “The Office of Prescription Drug Promotion (OPDP) of the U.S. Food and Drug Administration (FDA) has reviewed the Kim Kardashian Social Media Post (social media post) (2015-0069-01) 1 for DICLEGIS (doxylamine succinate and pyridoxine hydrochloride) delayed-release tablets, for oral use (DICLEGIS) submitted by Duchesnay, Inc. (Duchesnay) under cover of Form FDA 2253.” 2015-0069-01 identifies the image shown here.
So, sorry for the misleading original title of this post. But the last point is still interesting: FDA seems to be acting much faster when it comes to social media drug promotion violations than to TV (or print) violations.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




