At the end of June, the FDA sent a Notice of Violation (NOV) letter to Dr. C (you can find his name in the letter), Director, Regulatory Affairs Advertising and Promotion at Gilead Sciences, citing a sponsored link on Google for VIREAD (you can find the letter here).
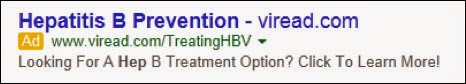
Here’s the ad in question:
Unless Dr. C (he has a PharmD degree) has been hiding under a “regulatory rock” since 2009, it’s difficult to imagine that he would have approved such an ad. Because as WE all know, FDA views such ads, which include the drug brand name AND indication but no fair balance safety information, as violative of the FD&C Act.
Anyone, such as Dr. C, who is a regulatory authority — he’s worked in a regulatory capacity for several pharma companies since 2004 — should be aware of FDA’s “received precedent” on this type of ad (i.e, 14 warning letters issued in April 2009).
VIREAD is associated with serious risks and includes a black box warning. To advertise such a drug without providing any mention of such risk is unconscionable.
But there are even more serious problems with this ad, that, IMHO, should get Dr. C in hot water with Gilead’s CEO!
According to the FDA letter, the ad “misleadingly suggests that Viread is safe and effective for use in the prevention of hepatitis B. According to the PI, ‘Viread is indicated for the treatment of chronic hepatitis B in adults and pediatric patients 12 years of age and older’ (bolded emphasis added). The approved labeling for Viread does not provide instructions for, or otherwise indicate that Viread will be safe and effective if used for the prevention of hepatitis B. Information sufficient to demonstrate that Viread is safe and effective for this new intended use has not been submitted to FDA in an application.”
Not only that, FDA cites these additional regulatory failures:
- “The sponsored link fails to present the established name for Viread (tenofovir disoproxil fumarate), despite the requirement to do so in direct conjunction with the proprietary name.”
- “A copy of the Viread sponsored link was not submitted to OPDP under cover of Form FDA-2253 at the time of initial publication as required by 21 CFR 314.81(b)(3)(i).”
Which leads me to ask: “Where the heck was Dr. C when Gilead marketers decided to run this ad?” Was he on vacation? Perhaps an aide approved the ad. Perhaps the ad was not submitted to his department and he can pass the buck on to the marketing team. Perhaps Gilead’s interactive agency decided to create the ad campaign without telling Gilead or Dr. C.
Whatever the reason, the buck stops with the Director of Regulatory Affairs. If he was left out of the loop in this case, then it is easy to understand why medical-legal executives are not as cooperative as some pharma marketing people would like them to be. The name of the regulatory person is on the FDA letter, not the marketing manager’s.
But, wait! FDA is requesting only that Gilead “immediately cease violating the FD&C Act” and submit a written response to the letter on or before July 11, 2014, stating whether it intends to comply with this request, “listing all promotional materials (with the 2253 submission date) for Viread that contain presentations such as those described above, and explaining your plan for discontinuing use of such materials.”
In other words, Gilead is basically free to continue running the ad until at least July 11, 2014 — two weeks after receiving the FDA letter. BTW, the ad probably ran for a number of weeks BEFORE the FDA even sent the letter.
For these reasons, I don’t expect that Dr. C will lose his job. In fact, he may get a bonus and hang the FDA letter on his wall as a trophy!








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




