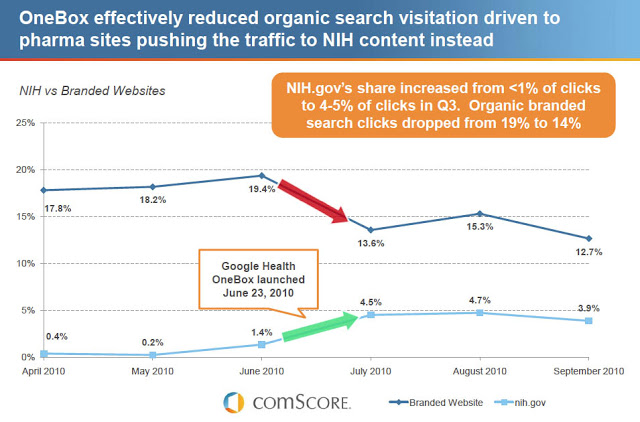
Last year I posted data that showed Google’s OneBox Rx “Ads” steal clicks from organic branded Rx search results (see chart below).
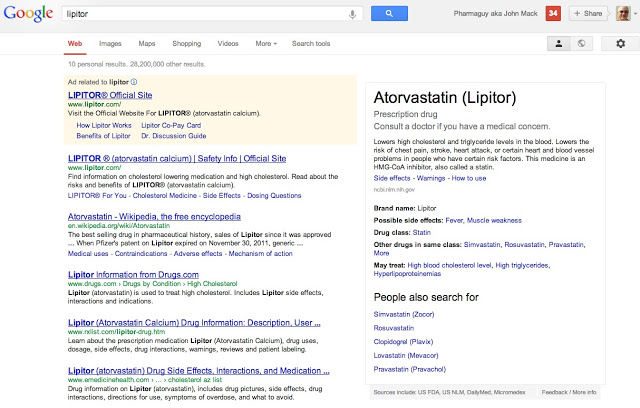
I just learned that Google has gone one step further. Instead of a small adword-type box near the top of the search results page (i.e., BELOW the paid ad, if any), there is a large box on the upper right-hand portion of the page that includes a lot more information. The following screen shot illustrates the size and placement of the new “sidebar” drug information box on the search results page for Lipitor (click the figure for an enlarged view).
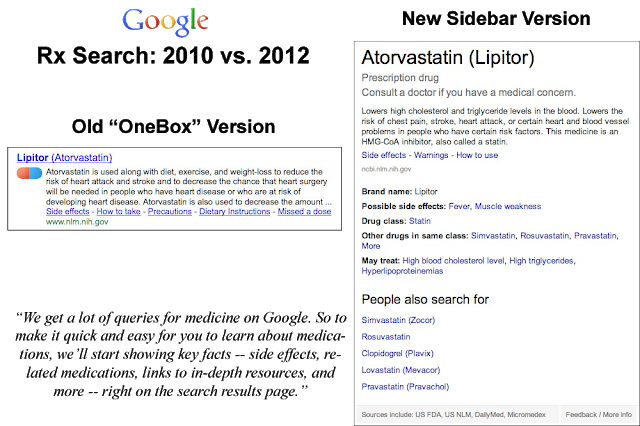
Here’s how this version (right) compares with the “old” OneBox version (left):
Google says “this data comes from the U.S. FDA, the National Library of Medicine, and the Department of Veterans Affairs, among others” (see here).
Lacks Lifestyle Information
Aside from the difference in size and page location, these two versions have one extremely notable (IMHO) difference; i.e., the old version includes the phrase “used along with diet, exercise, and weight loss” whereas the new version does NOT include any lifestyle info, which FDA and PhRMA recommend to be included in direct-to-consumer (DTC) ads on TV and in print (see DTC Guideline #12) .
The source of the information in both versions is said to be the National Library of Medicine (NLM), which has this description of Atorvastatin:
“Atorvastatin is used along with diet, exercise, and weight loss to reduce the risk of heart attack and stroke and to decrease the chance that heart surgery will be needed in people who have heart disease or who are at risk of developing heart disease. Atorvastatin is also used to decrease the amount of cholesterol (a fat-like substance) and other fatty substances in the blood. Atorvastatin is in a class of medications called HMG-CoA reductase inhibitors (statins). It works by slowing the production of cholesterol in the body to decrease the amount of cholesterol that may build up on the walls of the arteries and block blood flow to the heart, brain, and other parts of the body (see here).”
Why is the lifestyle information missing in the new version of Rx search results? Why has Google decided to switch to this new version in the first place?
As you know, I have a conspiracy theory that involves Google and those infamous 14 NOV letters concerning search ads FDA sent to pharma companies in 2009 (see here). According to that theory, FDA is forcing Google to implement these Rx information boxes because it was a “bad boy” by encouraging illegal drugstore paid ads to be included on search pages. FDA “punished” Google with the 14 letters, cutting off lucrative drug company advertising. The result of this was the “OneBox”, 2010 version, which competed with drug company search engine referrals as noted above. My thinking is that drug companies complained to the FDA and Google about this and that resulted in the 2nd generation, 2012 version of the Rx information box devised by Google.
The new box, however, is much bigger and more visible than the old version. This could not have been acceptable to the drug industry without further concessions such as (1) eliminating the lifestyle information, which PhRMA guidelines only suggest for TV and print ads anyway, and (2) making the info box look LESS like a competing paid search ad and moving it out of the area typically reserved for paid and organic search results.
It is unknown whether of not the new format will alleviate the problem of competition with organic and paid search ads illustrated in the chart at the beginning of this post. I’d be interested in hearing more opinions about that and seeing some data.
Will It Spur New, Favorable FDA Guidelines?
If indeed FDA, Google, and Pharma are collaborating on what information to include in the box and and where it should be located on the Rx search result page, then we may be closer than we think to FDA coming out with new favorable guidance for drug industry search ads; e.g., officially sanctioning this “beta” version of Rx adwords shown below:
That would be the best concession pharma could hope for.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




