“I could strangle that girl from Google,” said a friend of mine after a presentation by a Google operations specialist at the recent eyeforpharma eCommunications and Online Marketing conference held in Philadelphia. Of course, this was not a literal terroristic threat, just an expression of frustration by a long-time industry advocate.
What did the “Girl from Google” (GfG) say that upset this person so much?
The “Girl from Google” (GfG) was up just before my presentation and the title of her talk was “The Importance of Interactivity: How multimedia technologies will change the way you Connect with Consumers & Physicians.”
It was actually a nice presentation but it could have benefited from a little legal/regulatory review beforehand. GfG was one of those presenters who have little or no experience working within or for the pharmaceutical industry. Her bio in the conference brochure says only that she has worked with Google for three years (congrats on the stock options!) and graduated with a Masters Degree form the University of Southern California (party on!). I estimate that GfG joined Google straight from school.
I had only one little problem with her presentation.
As an example of how Google Health can help pharmaceutical companies engage consumers online, GfG used as an example a fictitious drug — let’s call it Xenaxa — indicated for treatment of osteoporosis. The target consumer was a fifty year old woman concerned about osteoporosis. She does a search on Google Health and finds an AdWord for Xenaxa.
Would you believe that GfG’s example used an Adword exactly like the Lunesta AdWord I talked about a few days ago on this blog (see “Lunesta, Google, and bAdWords“)? Just like that Lunesta AdWord, the AdWord GfG used in her example included both the trade name of the drug (in the URL) and its indication.
As with the Lunesta AdWord, this example also violates FDA regulations, IMHO. Harry Sweeney agreed. I asked the audience what they thought. One person from a pharmaceutical company, perhaps playing the devil’s advocate, contended that the ad may pass muster with the FDA because the package insert or brief summary is “one or two clicks away.” His argument was that without specific guidance from the FDA, no one knows what is correct in this case.
One Click Away?
The “one click away” defense does not apply here. FDA says it’s OK on an Rx product Web site to merely provide a link to the package insert or brief summary. In that case there is no need to provide that information on the same page that mentions the drug name and its indication.
Thus, an AdWord could be said to comply with the “one click rule” only if within the AdWord text there was a direct link to the package insert (PI) or brief summary. In the example that GfG used, there was only a link to www.xenaxa.com — the product Web site, not the PI. Presumably, the user would have to find the link to PI once on the Xenaxa Web site. I don’t think two clicks would pass muster with the FDA (more on that in future posts to this blog).
It is a shame that the FDA does not have any guidance for the industry as far as Internet advertising is concerned. This means that marketers can use lack of guidance as a defense for sneaking in ads that push the envelope. What are the chances that the FDA would ever notice. These ads are fleeting, here today, gone tomorrow!
However, that should not stop the industry from developing its own Internet DTC advertising guidelines, which it promised to do when PhRMA released DTC guidelines for broadcast DTC.
In this case, however, I think the issue is clear. Any AdWord that mentions a product by name plus the indication, must also include a direct link to the PI within the ad itself.
What do you think?
Consider the Lunesta Adword below:
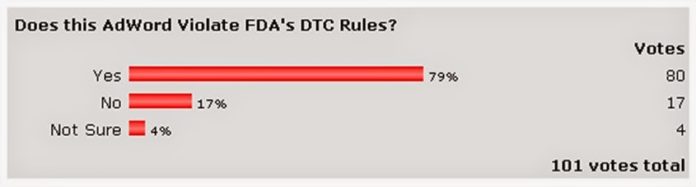
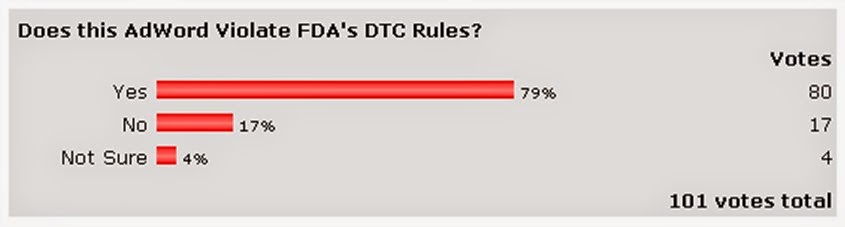
| Does this AdWord Violate FDA’s DTC Rules? Results of poll: |
What the “Girl from Google” just doesn’t see is what the fuss is all about. “We don’t think it’s a problem and it’s not our roll to enforce the law.” This attitude is absolutely stunning. She claims Google is neutral, but as I pointed out before, Google does intervene when it thinks EU laws about DTC advertising are being violated. Why not when US laws are involved?
Update: Much has happened in the legal/regulatory world that has proved me right. Namely, FDA sent 14 warning letters to pharma companies in April, 2009, about Google AdWords similar to the ones mentioned her and promoted by the Girl from Google at this conference.









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




