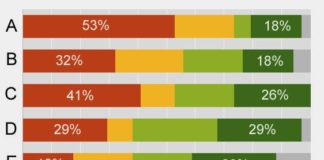
The Union of Concerned Scientists (UCS) recently released survey results from 997 FDA scientists that demonstrate pervasive and dangerous political influence of science at the agency. Thirty-four percent (34%) of respondents were consumer safety officers and 62% were top-level officials.
According to the UCS press release:
“Forty percent of respondents fear retaliation for voicing safety concerns in public…More than a third of the respondents did not feel they could express safety concerns even inside the agency. Nearly one-fifth (18.4 percent) said that they ‘have been asked, for non-scientific reasons, to inappropriately exclude or alter technical information or their conclusions in a FDA scientific document.'”
One FDA scientist that is not afraid to speak out is Dr. David Graham, part of the Food and Drug Administration’s drug safety office, who, according to an article in the New York Times (“Approval of Antibiotic Worried Safety Officials“), “wrote in a message dated June 16 that the agency’s approval of Ketek, an antibiotic made by Sanofi-Aventis that is also known as telithromycin, was a mistake.”
I covered the Ketek safety issue in a previous post to this blog (see “Celsius 3014: Ketek, Drug Safety, & Bioterrorism“) in which I suggested that the FDA’s approval was pressured by the political demands of the Department of Homeland Security. What I thought was a conspiracy theory may yet turn out to be more fact than conjecture!
This all has to do with “post-market surveillance” and NOT with the balance of benefit vs risk as some pundits have claimed. It’s difficult to make a risk-benefit calculation if you don’t have reliable data and that is what is at the heart of the Ketek case. According to Graham:
“We don’t really know if the drug works; no one is claiming it works better than other, safer drugs; and weÂre flying blind as far as safety goes, except for our own A.D.R. data that suggests telithromycin is uniquely more toxic than most other drugs.”
[It should be noted that some critics of Dr. Graham attack his character rather than his opinions. One example: “David Graham who is to rational evaluation of the risks and benefits of medicine what Hezbollah is to advancement of peace in the Middle East…”
Q: How do you distinguish a conservative from a liberal these days?
A: The conservative, eg, Karl Rove, criticizes the character of an opponent, the liberal criticizes the opponent’s point of view.]
ADR data is notoriously unreliable, but the safety data from post-marketing surveillance studies done in France and Latin America (ie, “Study 3014”)–upon which basis Ketek was approved–may be even more unreliable.
“For F.D.A. to refer to its being reassured by postmarketing data from Latin America and Europe as a basis for declaring ‘Ketek is safe’ is in my opinion a great abuse of such surveillance data,” Dr. Graham wrote.
Political influence over science is one thing, but mixing politics and bad science is a formula for disaster. Maybe Ketek is not the most dangerous drug on the market, but it could be the canary in the minefield that is the state of postmarketing surveillance in this country (for more on this topic, see “Spinning Bad News about FDA & Drug Safety” and “FDA Paralyzed: Who Will Protect Us?”
For additional blog posts about the FDA, see:
How to Keep Up
To keep in the loop with posts made to Pharma Marketing Blog, you could subscribe to the newsletter and/or online forum or you can receive notice by email whenever a post is made to Pharma Marketing Blog by subscribing below.Enter your Email Address









![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)