Currently, the chart includes only those requirements with specific statutory dates set by Congress. “Additional deliverables will be added to the chart over time as FDA continues to advance its implementation planning efforts,” says the FDA.
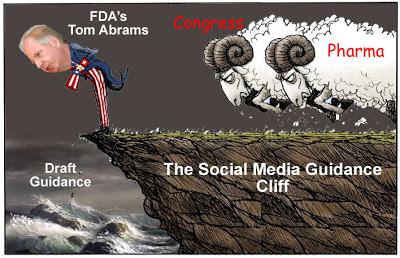
Of course, I am laser-focused on ONLY one “deliverable”: Draft guidance describing FDA policy regarding Internet promotion, including social media, of medical products regulated by FDA. Congress has set 9-JULY-2014 as the deadline for this guidance.
In order to be in step with current Congressional media jargon, I refer to this date as FDA’s “Social Media Guidance Cliff” although this “cliff” — and, IMHO, the “Fiscal Cliff” as well — is more like a slippery slope (see here).
If you are a regular reader of Pharma Marketing Blog, you know that I am not content to report the news. I also attempt to influence the people who make the news and goose things along by being a bit provocative.
So, I have decided to send an email to the person listed as the contact for this deliverable — Megan Clark Velez, MPH, Office of the Commissioner — and ask her for a bit more transparency. I decided to make this an “open” email by publishing it below in the hopes that some of you may also contact Ms. Velez with your own questions. Her contact info:
Megan Clark Velez, MPH
Food and Drug Administration
10903 New Hampshire Ave., WO1, Rm. 4316
Silver Spring, MD 20993
Phone 301-796-4830
Email: megan.velez@fda.hhs.gov
My email to Megan Clark Velez:
Dear Ms. Megan Clark Velez,
Your email address was listed as the contact on the FDASIA-TRACK site for draft guidance regarding internet promotion, the deadline for which is 7/9/2014 (I call this the “Social Media Guidance Cliff”).
The deadline seems far off, but FDA has supposedly been “serious” about issuing this guidance since the November, 2009, public hearing.
Maybe you can answer a few questions:
FDA has said that there may be several guidances in this category. “What had been envisioned as a massive, all-encompassing guidance on Internet promotion is being retooled as multiple guidances to address specific issues in the online realm,” Tom Abrams said (see http://bit.ly/SgS21f). Recent guidance for unsolicited requests for off-label info, for example, included some guidance for responding to such requests via social media. Will there be other guidance documents like this issued BEFORE the “Social Media Guidance Cliff”?
If not “hidden” within other guidances, will there be any guidance SPECIFIC for social media prior to the deadline?
If such “mini” guidances ARE issued prior to the “Social Media Guidance Cliff,” what question/area posed at the November, 2009, hearing will the first such guidance document address?
Whatever you can tell us — the general public and the pharma industry — about what the FDA is doing behind closed doors and progress being made to develop these guidances will be appreciated.
Thank you for your attention to this matter and your much anticipated response!
John Mack, Editor & Publisher
Pharma Marketing News/Pharma Marketing Blog








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




