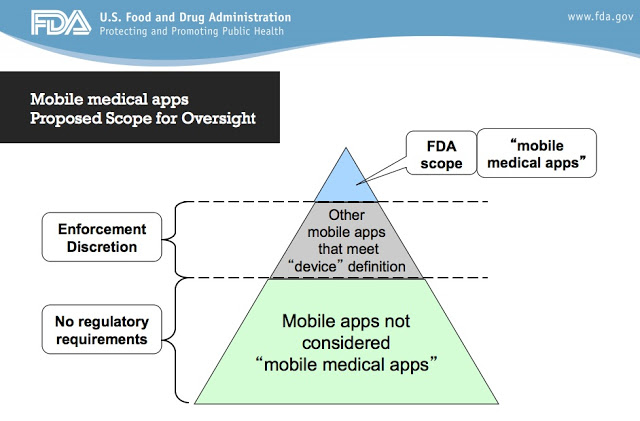
“Consider the ‘Mobile medical apps Proposed Scope for Oversight’ issued by CDRH [FDA’s Center for Devices and Radiological Health],” said DrugWonk Peter Pitts (here). “It’s a pyramid divided into three parts”:
Pitts describes these parts of the pyramid:
- The top of the pyramid includes mobile medical apps that are traditional medical devices or a part or an extension of a traditional medical device. Clearly within the scope of being regulated as medical devices.
- The middle section includes patient self- management apps and simple tracking or trending apps not intended for treating/adjusting medication. This is the area, as defined by CDRH, for enforcement discretion
- The bottom section are devices that are not deemed “mobile medical apps” and, as such, have no regulatory requirements.
I notice that many reporters and drug industry people refer to “mobile medical apps” when describing apps that are CLEARLY at the bottom of CDRH’s pyramid. An example is the article titled “11 Super Mobile Medical Apps” that offers these examples of apps, which clearly should NOT be called MMAs:
“Among the innovative mobile medical apps we found is one that lets doctors use interactive diagrams to show patients what’s happening with their bodies, where procedures will be done, and exactly what will happen during different procedures. Alternatively, patients can use this app to get doctors to provide detailed visual answers to their questions.”
Unfortunately, FDA’s co-optation of “mobile medical app” to refer to health apps requiring regulation as medical devices confuses the discussion, which up until now used the term to describe any health-related app that a physician or patient might use. This may be why PhRMA and other drug industry spokespeople are so fearful of FDA regulations hampering innovation within the “mobile health app” arena (see, for example, “FDA Mobile Regulatory Fear Mongering by PhRMA“).








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)