According to a Pharmalot headline, FDA’s Tom Abrams — who heads the Office of Prescription Drug Promotion — promises that “Long-Awaited Social Media Guidance Is Coming” (see here).
Despite Ed Silverman’s valiant attempts to pin Abrams down to a specific time frame, all he was able to get from Abrams was “It’s hard to anticipate the time, because there are many steps involved.”
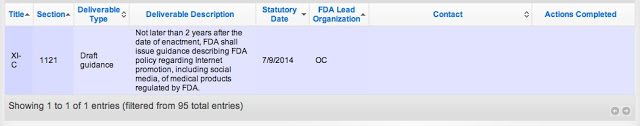
Abrams wouldn’t even commit to the July 9, 2014, deadline mandated by Section 1121 in FDASIA. “Our intention is to make that,” said Abrams. “Any chance you can make it sooner?,” asked Silverman. “We are striving to make it as soon as possible,” said Abrams.
Abrams emphasized the “many steps” involved in the process and that his peeps “are working very, very thoroughly and very hard – people are putting in extra hours.”
So what progress has FDA made? What actions have been completed? Surely, after all the extra hours there must be some progress to report.
According the FDASIA-TRACK site, through which FDA “will communicate its progress towards accomplishing the requirements of the FDA Safety and Innovation Act,” the agency has completed NO actions with regard to “issuing guidance describing FDA policy regarding Internet promotion, including social media, of medical products regulated by FDA.” Here’s the section of the FDASIA-TRACK showing the lack of progress as of March 29, 2013:
I stand by my prediction that the FDA will prevaricate and delay the issuance of social media guidance until it reaches the very edge of the July, 2014, “Social Media Guidance Cliff” and Beyond! For more on that, see here.








![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




