FDA has just released two guidance documents that finally address major issues discussed at the November 2009 public hearing:
- How to present both benefit and risk information within a “promotion” of their FDA-regulated medical products on electronic/digital platforms that are associated with character space limitations, specifically on through social media such as Twitter and through online paid search (e.g., “sponsored links” on search engines such as Google and Yahoo); and
- How to respond to misinformation related to a firm’s own FDA-approved or -cleared products when that information is created or disseminated by independent third parties on the Internet or through social media, regardless of whether that misinformation appears on a firm’s own forum or an independent third-party forum or website.
Don’t bother reading the first one about posting promotions via Twitter and other venues with space limitations. FDA is NOT approving the “one-click rule,” which started this whole mishegoss about regulating pharma marketing via social media (see Death of the One-Click “Rule” or “Received Precedent” or Whatever!). FDA essentially says, too bad, you have to include everything — benefits AND major side effects — in the space allotted. Plus, you also have to link to more detailed information about side effects.
Essentially, unless you do reminder ads (like Novo Nordisk’s Branded (Levemir) Sleazy Twitter Spam), you can forget about Twitter to promote your drug. And be careful about those paid Google ads too — FDA did NOT mention Google’s beta format that allows for more text.
The guidance about correcting misinformation is more interesting and should satisfy the pharmaceutical industry — at least those companies that care about devoting resources to correcting misinformation on social media and the Internet in general.
One interesting take away actually has nothing to do with correcting misinformation per se. It has to do with hosting online discussion forums about Rx products.
Take a look at Example 4:
“A firm hosts a discussion forum about its drug’s or device’s FDA-approved use on its corporate website and does not participate in the discussion, but it does monitor the forum for profanity and obscenity. The forum includes an overarching clear and conspicuous statement that the firm did not create the content of the forum. The firm is not responsible for the information that is posted by independent third parties and can, if it so chooses, correct misinformation according to this guidance.”
Does this open a door through which pharma companies will walk? Not sure. I do not know of any example of pharma hosting a discussion about its products on its website, although there are probably several that host unbranded discussions (e.g., Pfizer’s Get Old site; read “Pfizer’s Social Media Initiatives are Getting Old“).
If a pharma company DID host such discussions, I’m sure it would screen all posts BEFORE they are uploaded to the site. Consequently, there would never be any visible “misinformation” to correct. Also, what pharma company would host such a discussion at “NOT [my emphasis] participate in the discussion”? That would negate the whole premise of social media “conversation” about products that the drug industry would like to have, if you believe their PR departments (e.g., read “Gefällt Mir! Boehringer Ingelheim Invites Two-Way Communication Via Social Media“). Pfizer, for example, participates in the Get Old discussions, but these discussions are not about its products.
In any case, FDA includes this caveat: “However, a firm’s control over, involvement with, or influence over a product-related communication, even when generated by a third party, may result in the firm being responsible for the information as a promotional communication. Thus, firms might be responsible for UGC that they solicit or influence, regardless of the forum.”
I think the key word here is “solicit.” I’ll have to search my treasure trove of FDA regulatory documents to see how FDA explains what it means by “solicit.”
Correcting Wikipedia Misinformation
In previous comments to the FDA, the pharma industry is demanded that any edits it makes to Wikipedia drug articles should NOT be subject to FDA regulations at all.
“FDA,” said PhRMA in comments submitted to FDA (see The Pros and Cons of Pharma Editing Wikipedia Articles), “should confirm formally that, while it is not possible for manufacturers to monitor or correct all inaccurate information about their products on the Internet, such corrections by manufacturers in response to inaccurate postings will not be considered promotional labeling. FDA’s adoption of such a policy would thereby allow manufacturers to correct inaccurate information about their medicines on the Internet or social media (e.g., Wikipedia, Sidewiki, blogs, or other websites) if they should become aware of such information.”
In its guidance, FDA more or less grants this request:
“When a firm voluntarily undertakes the correction of misinformation in a truthful and non-misleading manner pursuant to the recommendations in this draft guidance, FDA does not intend to object if these voluntary corrections do not satisfy otherwise applicable regulatory requirements, if any.”
FDA specifically refers to Wikipedia in Example 11 of the guidance document:
“A firm finds a webpage about its product that was written by an independent third party on an Internet-based, interactive, collaboratively edited encyclopedia. The firm may choose to contact the author of the webpage and provide corrective information to the author.”
Obviously “an independent third party on an Internet-based, interactive, collaboratively edited encyclopedia” is Wikipedia!
Pharma companies may be able to contact the author of “misinformation” on Wikipedia by leaving a message on the author’s talk page or perhaps suggesting edits on the discussion page of the article in question, but these suggestions are likely to be ignored.
There’s a better way, which I wrote about in Pharma Marketing News. In that article (find it here), I wrote about the “right way” to correct Wikipedia articles:
Recall that back in June, 2012, Dr. Bertalan Meskó (@Berci), in an open letter to pharma, urged the pharmaceutical industry to employ Wikipedia editors and thus “funnel [their] vast resources” to help (see “Pharma Urged to Employ Wikipedia Editors“).
“Based on the pretty negative past encounters between pharma employees and Wikipedia editors (pharma employees trying to edit entries about their own products in a quite non-neutral way), we advise you to employ a Wikipedia editor if you want to make sure only evidence-based information is included in entries about your own products,” said Berci in 2012 (see here).
“Appointing someone from within your company as a ‘spokesperson’ in Wikipedia who would perform all edits on behalf of the company is an excellent way to update those entries,” said Berci.
You would think that the pharmaceutical industry would have jumped at the chance to establish a liaison with Wikipedia to help edit articles about their products. At least one pharma company, in fact, did seem to endorse the idea, at least in principle.
Boehringer Ingelheim (BI) responded to Berci via Twitter: “We look for patient safety issues & react. Its important to stick to Wikipedia policies too, so all transparent.” But when asked by Berci if BI had posted anything online about this, BI responded “No at this point in time we have not….yet.” Nevertheless, Berci remains optimistic.
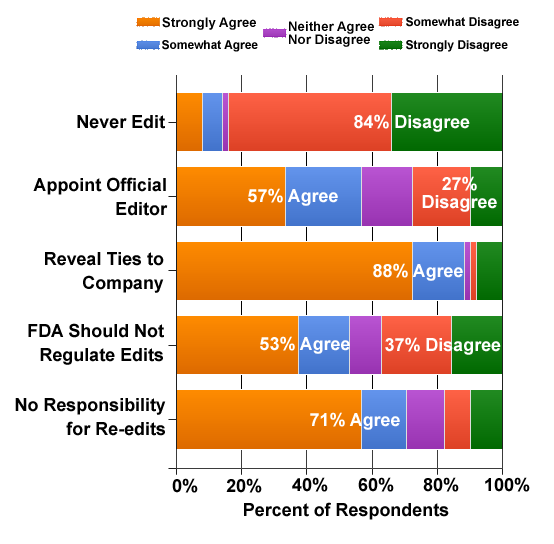
In a PMN survey, 57% of respondents agreed that pharma companies should appoint an official Wikipedia editor (see the figure below and survey summary here).
The public has 90 days to submit comments to the FDA about these guidances.










![6 Digital Tools at the Center of Healthcare Digitalization [INFOGRAPHIC]](http://ec2-54-175-84-28.compute-1.amazonaws.com/pharma-mkting.com/wp-content/uploads/2021/04/6DigitalTools_600px-100x70.jpg)




